Vascular Intervention // Coronary
Drug-Eluting Stent System
Orsiro
Clinically proven
Extensive clinical program*

*status as of Feb 2017
Outstanding clinical results even in challenging subgroups
Orsiro has demonstrated consistently low target lesion failure (TLF) in all-comers trials compared to major modern drug-eluding stents (DES).
The new benchmark for DES
BIOFLOW-V 12-month clinical outcomes compared to Xience
In a post-hoc analysis of pooled patient-level data from three RCTs, Orsiro achieved a 96.9% probability of superiority* on TLF rate versus Xience.10

Highly deliverable
Designed for challenging cases, the Orsiro stent system provides better push and easier cross with a lower crossing profile.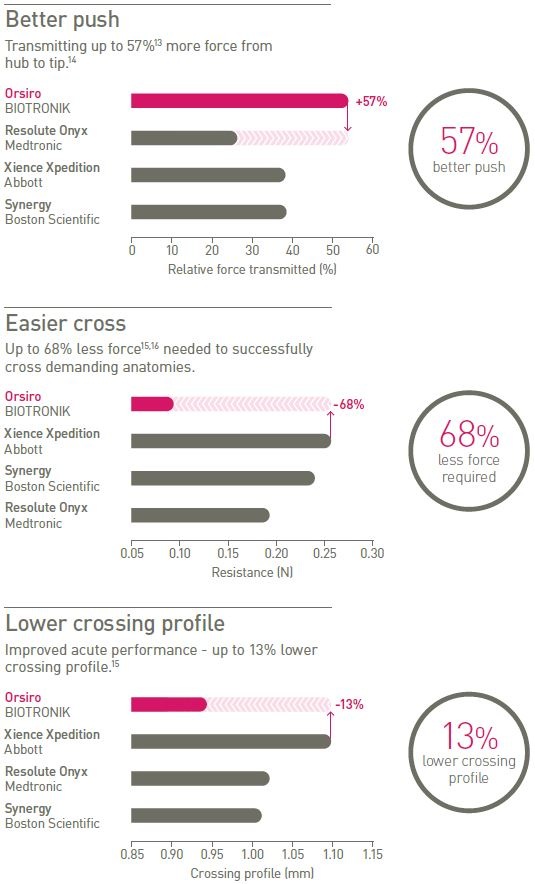
Ultrathin 60 µm struts
Thinner struts make the difference

 Orsiro
OrsiroIndicated for discrete de novo stenotic lesions and in-stent restenotic lesions.*
Technical Data
| Stent | |
|---|---|
| Stent material | Cobalt chromium, L-605 |
| Passive coating | proBIO (Amorphous Silicon Carbide) |
| Active coating | BIOlute bioabsorbable Poly-L-Lactide (PLLA) eluting a limus drug |
| Drug dose | 1.4 μg/mm2 |
| Strut thickness | ø 2.25 - 3.0 mm: 60 μm (0.0024"); ø 3.50 - 4.0 mm: 80 μm (0.0031") |
| Delivery System | |
|---|---|
| Catheter type | Rapid exchange |
| Recommended guide catheter | 5F (min. I.D. 0.056") |
| Lesion entry profile | 0.017" |
| Guide wire diameter | 0.014" |
| Usable catheter length | 140 cm |
| Balloon material | Semi Crystalline Polymer material |
| Coating (distal shaft) | Hydrophilic coating |
| Marker bands | Two swaged platinum-iridium markers |
| Proximal shaft diameter | 2.0F |
| Distal shaft diameter | 2.6F: ø 2.25 - 3.5 mm; 2.8F: ø 4.0 mm |
| Nominal pressure (NP) | 8 atm |
| Rated burst pressure (RBP) | 16 atm |
Compliance Chart
| Balloon Diameter x Length (mm) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Pressure | atm4 | 8 | 8 | 8 | 8 | 8 | 8 | ||||||||||||
| (NP) | ø (mm) | 2.25 | 2.50 | 2.75 | 3.00 | 3.50 | 4.00 | ||||||||||||
| Rated Burst Pressure | atm4 | 16 | 16 | 16 | 16 | 16 | 16 | ||||||||||||
| (RBP) | ø (mm) | 2.50 | 2.77 | 3.05 | 3.33 | 3.88 | 4.44 | ||||||||||||
Ordering Information
| 2.25 | 364469 | 364475 | 364481 | 364487 | 364499 | 364505 | 364511 | 391234 | 391238 | |||||
| 2.50 | 364470 | 364476 | 364482 | 364488 | 364500 | 364506 | 364512 | 391235 | 391239 | |||||
| 2.75 | 364471 | 364477 | 364483 | 364489 | 364501 | 364507 | 364513 | 391236 | 391240 | |||||
| 3.00 | 364472 | 364478 | 364484 | 364490 | 364502 | 364508 | 364514 | 391237 | 391241 | |||||
| 3.50 | 364473 | 364479 | 364485 | 364491 | 364503 | 364509 | 364515 | 391018 | 391020 | |||||
| 4.00 | 364474 | 364480 | 364486 | 364492 | 364504 | 364510 | 364516 | 391019 | 391021 | |||||
Contact
1 von Birgelen et al. Very thin strut biodegradable polymer everolimus-eluting stents versus durable polymer zotarolimus-eluting stents in all-comers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. The Lancet 2016. 10.1016.S0140-6736(16)31920-1 and presentation at TCT 2016;
2 TLF as a composite of cardiac death, target vessel-related myocardial infarction, or clinically indicated target lesion revascularization;
3 Pilgrim et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularization (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. The Lancet 2014.10.1016/S0140-6736(14)61038-2;
4 TLF as a composite of cardiac death, target vessel myocardial infarction, and clinically indicated target lesion revascularization;
5 Jensen et al. Randomized comparison of a sirolimus-eluting Orsiro stent with a biolimus-eluting Nobori stent in patients treated with percutaneous coronary intervention: Rationale and study design of the Scandinavian Organization of Randomized Trials with Clinical Outcome VII trial. 10.1016/j.ahj.2015.05.009;
6 Target Lesion Failure as a composite of cardiac death, myocardial infarction (not related to other than index lesion), or taret lesion revascularization;
7 Piccolo R. Biodegradable polymer Sirolimus-eluting stents vs. Durable polymer Everolimus- eluting stents in patients with STEMI: Two-year follow-up of the BIOSCIENCE oral presentation, EuroPCR 2016;
8 Definite or probable stent thrombosis per ARC definition;
9 Preliminary analysis based on non locked data – Ton Slagboom, poster presentation, presented at TCT, November 2016;
10 Kandzari et al. Ultrathin Bioresobable Polymer Sirolimus-Eluting Stents versus thin durable Polymer Everolimus-eluting stents in patients Undergoing Coronary Revascularization (BIOFLOW-V): a randomized trial, The Lancet 2017;
11 Adapted from SCAAR data (August 24th, 2016) http://www.ucr.uu.se/swedeheart/99-scaar/forskning-scaar;
12 Compared to other DES included in SCAAR at five years;
13 Compared to Resolute Onyx;
14 The stent system is advanced through a model, to a point of blockage (simulating a total occlusion). The force at the proximal hub and the blockage is measured. Pushability is the force transmitted along the length of the catheter. IIB(P)31/2015 – IIB(P)85/2014-2;
15 Compared to Xience Xpedition;
16 The stent system is advanced through a stenosis model. Crossability is the mean resistance (mean force) registered by the stenosis during the complete passage of the stent delivery system. IIB(P)31/2015 – IIB(P)85/2014-2;
17 Stefanini GG, Taniwaki M, Windecker S. Coronary stents: novel development, Heart doi:10.1136/heartjnl-2012-303522;
18 Foin et al. Impact of stent strut design in metallic stents and biodegradable scaffolds. Int J Cardiol.2014 Dec 20;177(3):800-8;
19 Compared to Xience Expedition;
20 Expanded 3.0 mm diameter stents are radially compressed (15% of Ø) along full length. The force required to compress the stent is radial strength. BIOTRONIK data on file.
Synergy and Promus are registered trademarks of Boston Scientific; Resolute, Integrity, Resolute Integrity and Resolute Onyx are registered trademarks of Medtronic/Xience; Xience Prime and Xience Xpedition are registered trademarks of Abbott Cardiovascular Systems; Nobori and Ultimaster are registered trademarks of Terumo; BioMatrix is a registered trademark of Biosensors.
*Indication as per IFU.






