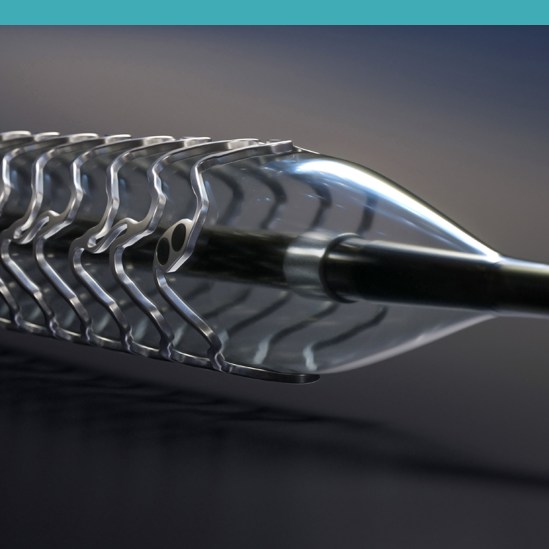
Product Details

First Clinically Proven Resorbable Magnesium Scaffold
No definite or probable scaffold thrombosis was observed with Magmaris.4 Previous generations showed no scaffold thrombosis up to 36 months.4

Fast Magnesium Resorption Time
~95 % of Magnesium resorbed at 12 months3

Compelling Safety Data2
The optimal combination of the strut design, electropolished smooth scaffold surface and biocompatible coating facilitate rapid endothelial coverage:
15 % better endothelialization at 28 days3,6

Robust Magnesium Backbone
Strong radial resistance:
No significant diameter change under increasing physiological pressure5

No recoil increase:
Conventional polymeric scaffold7 diameter decrease >20 % within 1st hour5

Metallic Scaffolds for Better Deliverability6
Magnesium allows for a smoother scaffold surface for:
- Better lesion crossing - up to 40 % lower lesion entry and crossing force5,6

- Better trackability in tortuous anatomy - 29 % less peak force5,6

The dual coated delivery system enables:
- Better pushability - 34 % more force transmitted from hub to tip5,6

Electropolishing Ensures Rounded Edges
Magmaris' smooth surface generates less resistance than the polymeric scaffold7
BIOSOLVE-I
- Prospective, multi-center, first-in-man trial testing DREAMS (Drug-Eluting Absorbable Magnesium Scaffold)
- Number of patients: 46
- Primary endpoint: TLF at 6 and 12 months

BIOSOLVE-II
- Prospective, multi-center, first-in-man trial to evaluate the safety and performance of Magmaris with a maximum of two de novo lesions in two separate coronary arteries
- Number of patients: 123
- In-segment LLL at 6 months

Technical Data
| Scaffold | |
|---|---|
| Scaffold material | Proprietary Magnesium alloy |
| Markers | Two tantalum markers at each end |
| Active coating | BIOlute bioabsorbable Poly-L-Lactide (PLLA) eluting a limus drug |
| Drug dose | 1.4 μg/mm2 |
| Strut thickness/width | 150 μm/150 μm |
| Maximum expandable diameter | Nominal Diameter +0.6 mm |
| Delivery System | |
|---|---|
| Catheter type | Rapid exchange |
| Recommended guide catheter | 6F (min. I.D. 0.070") |
| Crossing profile | 1.5 mm |
| Guide wire diameter | 0.014" |
| Usable catheter length | 140 cm |
| Balloon material | Semi-rystalline polymer |
| Coating (distal shaft) | Dual coated |
| Marker bands | Two swaged platinum-iridium markers |
| Proximal shaft diameter | 2.0F |
| Distal shaft diameter | 2.9F |
| Nominal pressure (NP) | 10 atm |
| Rated burst pressure (RBP) | 16 atm |
Compliance Chart
| Balloon Diameter x Length (mm) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Pressure | atm8 | 10 | 10 | ||||||||||||||||
| (NP) | ø (mm) | 3.00 | 3.54 | ||||||||||||||||
| Rated Burst Pressure | atm8 | 16 | 16 | ||||||||||||||||
| (RBP) | ø (mm) | 3.29 | 3.82 | ||||||||||||||||
Ordering Information
Contact

1 Indication as per IFU
2 BIOSOLVE-II
3 Pre-clinical trial. BIOTRONIK data on file
4 PROGRESS, BIOSOLVE-I, BIOSOLVE-II
5 BIOTRONIK data on file
6 comapred to polymeric scaffold (Abbott Absorb)
7 Abbott Absorb
8 1 atm = 1.013 bar