Product Details

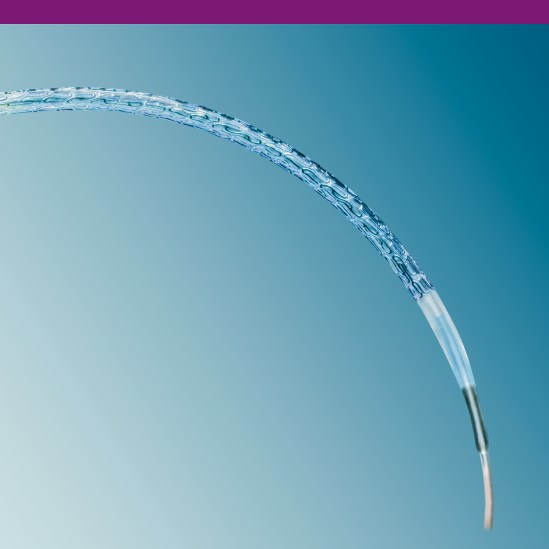
Advanced Stent Design
The PRO-Kinetic Energy stent design offers exceptional bending flexibility without compromising scaffolding or fatigue resistance. This advanced stent design allows for a smooth outer contour when bending without ridged transition zones.
Helical meanders give flexibility to the stent for excellent delivery and allow for a smooth crimped profile.
Wedge-shaped transitions at the stent ends allow for consistent scaffolding throughout the entire length of the stent.
Longitudinal connectors provide stability for optimal scaffolding and support without
sacrificing flexibility.
Ultra Thin Strut Design
Struts of only 60 μm1 result in exceptional flexibility and deliverability of the stent in even the most challenging anatomy.
Powerful Cobalt Chromium Alloy
Our advanced materials allow engineers to push the
limits of design with novel concepts for thinner struts
without compromising other aspects of the stent.
proBIO Silicon Carbide Coating
proBIO acts as a diffusion barrier, sealing the bare metal surface and reducing ion release. In vitro studies have shown up to a 96% reduction of allergenic metal ions4 when the stent surface is coated with silicon carbide.
By providing a barrier against ion release, the silicon carbide coating creates a surface that reduces platelet aggregation while facilitating endothelialization.3
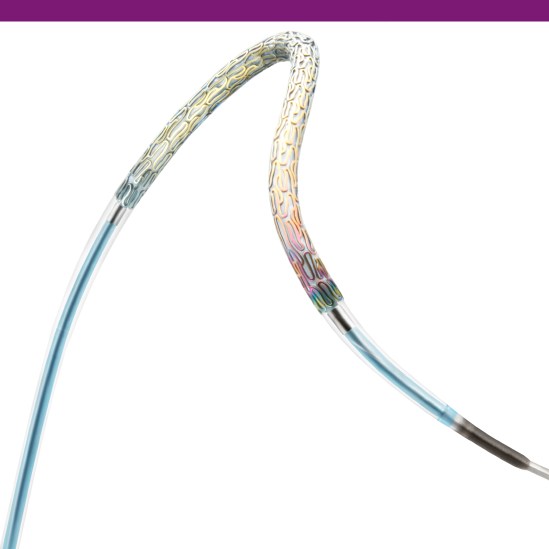
Innovative Stent Delivery System
Expect effortless deliverability from the stent delivery system built with Pantera PTCA balloon technology featuring an Enhanced Force Transmission (EFT) shaft and thinner materials for added pushability and trackability.
Enhanced Force Transmission (EFT) shaft improves kink resistance and pushability due to the gradual transition from the proximal to the distal part of the shaft.
Advanced Thermal Crimping
Advanced thermal crimping techniques were developed for PRO-Kinetix Energy to ensure secure stent retension forces as well as a smooth, low crossing profile (0.95 mm/0.037")5.
ENERGY Registry
- Prospective, non-randomized, multi-center, observational registry to evaluate the clinical performance of the PRO-Kinetic Energy BMS in a large real-world patient population in standard clinical care. Number of patients (n) 1,016
- Number of patients (n): 1,061
- Primary endpoint: MACE (Major Adverse Cardiac Events) - composite of cardiac death, clinically driven Target Lesion Revascularization, myocardial infarction and acute myocardial infarction (AMI) at 6 months
PEBSI RCT
- Prospective, multi-center, randomized, investigator-initiated trial to compare the efficacy and safety of the combined treatment of BMS plus DCB versus the conventional treatment (BMS only) in patients with STEMI within 12 hours of symptom onset. Baseline characteristics were similar in both groups
- Number of patients (n): 223
- Primary angiographic endpoint: 9-month late lumen loss (LLL)
Technical Data
| Stent | |
|---|---|
| Stent material | Cobalt chromium, L-605 |
| Passive coating | proBIO (Amorphous Silicon Carbide) coating |
| Strut thickness | ø 2.0 - 3.0 mm: 60 μm (0.0024"); ø 3.5 - 4.0 mm: 80 μm (0.0031"); ø 4.5 - 5.0 mm: 120 μm (0.0047”) |
| Delivery System | |
|---|---|
| Catheter type | Rapid exchange |
| Recommended guide catheter | Recommended guide catheter |
| Lesion entry profile | 0.017" |
| Guide wire diameter | 0.014" |
| Usable catheter length | 140 cm |
| Balloon material | Semi crystalline co-polymer material |
| Coating (distal shaft) | Hydrophilic coating |
| Marker bands | Two swaged platinum-iridium markers |
| Proximal shaft diameter | 2.0F |
| Distal shaft diameter | 2.5F: ø 2.0 - 3.5 mm; 2.8F: ø 4.0 - 5.0 mm |
| Nominal pressure (NP) | 9 atm |
| Rate burst pressure (RBP) | 16 atm (2.0 - 4.0 mm); 14 atm (4.5 - 5.0 mm) |
Compliance Chart
| Balloon Diameter x Length (mm) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Pressure | atm7 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | |||||||||
| (NP) | ø (mm) | 2.00 | 2.25 | 2.50 | 2.75 | 3.00 | 3.50 | 4.00 | 4.50 | 5.00 | |||||||||
| Rated Burst Pressure | atm7 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 14 | 14 | |||||||||
| (RBP) | ø (mm) | 2.33 | 2.59 | 2.83 | 3.12 | 3.42 | 4.07 | 4.65 | 5.11 | 5.63 | |||||||||
Ordering Information
Contact
1 2.0 - 3.0 mm stents
2 ISAR STEREO I & II; (I) Kastrati et al. 2001. Circulation. 103(23): 2816-21.; (II) Pache et al. 2003. J Am Coll Cardiol. 41(8): 1283-8.
3 Rzany A, Schaldach M. 2001. Progress in Biomedical Research 2001 May: 182-194.
4 BIOTRONIK data on file
5 3.0 mm stents
6 22 mm, 35 mm stents lenghts not available
7 1 atm = 1.013 bar
8 Size not licensed for sale in Canada.