Acticor 7 HF-T QP/HF-T
Simplified and optimized CRT for up to 9 years1
Heart failure is tough to live with and can be complex and time-consuming to treat and manage. It’s these real life challenges that Acticor 7 CRT-D systems are engineered to deal with. These new and smaller systems simplify treatment and therapy for up to 9 years1, and when used with BIOTRONIK Home Monitoring technology, they have been clinically proven to help prolong life further30.
Product Highlights
9 Years Longevity. 6 Years Warranty
Acticor CRT-Ds have an extended battery life of 9 years9, supported by a full 6-year warranty
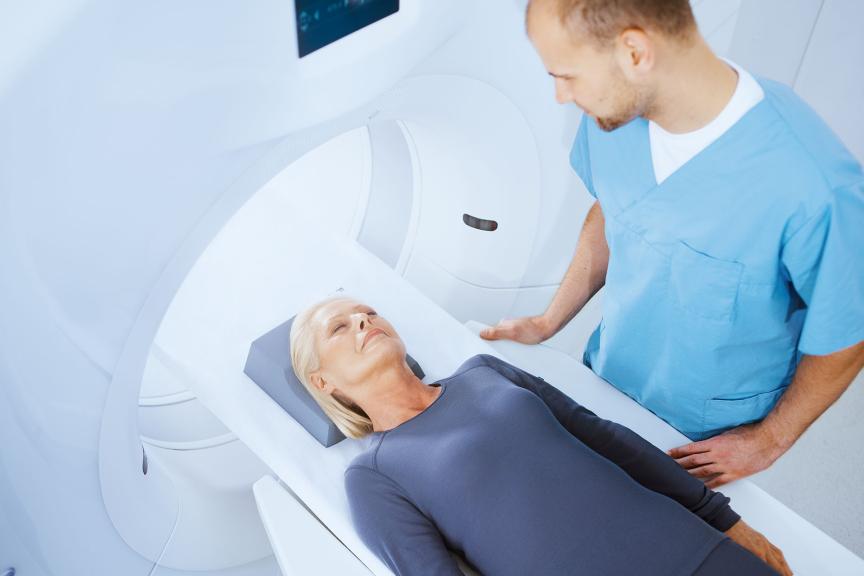
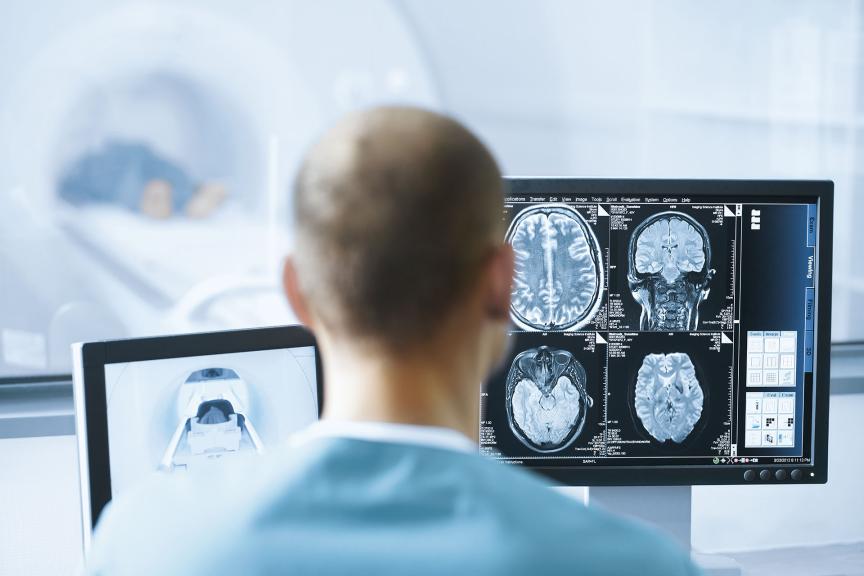
Full 3T MRI. Autodetected
All Acticor devices feature ProMRI, which gives full and uncompromised access to high-resolution MRI, with full-body scanning and no exclusion zone
Continuous CRT Optimization
Every minute, CRT AutoAdapt automatically adjusts to changes in the patient’s condition, thus providing continuous CRT adaptation and allowing for more personalized response.
Downloads & Related Links
Contact Us
References
1. Contoured housing; Acticor/Rivacor CRT-D QP: 60x75x10mm; 35ccm; CRT-D: 60x71.5x10mm; 33ccm; 2. as part of an MR conditional system; 3. Post-Market observation; interim-analysis, December 21, 2018. Data on file; 4. Device shape analysis, February 2019. Data on file; 5. Post-Market observation; interim-analysis, December 21, 2018. Data on file; 6. Post-Market observation; interim-analysis, December 21, 2018. Data on file; 7. Device shape analysis, February 2019. Data on file; 8. Post-Market observation; interim-analysis, December 21, 2018. Data on file; 9. Acticor/Rivacor HF-T QP, 9.3 years @ 60 ppm; RA 15%, RV/LV 100% pacing, RA/RV/LV @ 2.5 V/0.4 ms; 500 Ohms, 2 max. energy shocks/year. Data on file; 10. Acticor/Rivacor HF-T QP: 9.3 years @ 60 ppm; RA 15%, RV/LV 100% pacing, RA/RV/LV @ 2.5 V/0.4 ms; 500 Ohms, 2 max. energy shocks/year: Data on file vs. Medtronic 3T FBS; CLARIA (AMPLIA, COMPIA) MRI QUAD CRT-D SureScan: 6.8 years; Competitor device manuals as of Nov. 2018; 11. Lee DS et al. Evaluation of Early Complications Related to De Novo Cardioverter Defibrillator Implantation. Insights From The Ontario ICD database. J Am Col Cardiol 2010; 55:774-82; 12. Fact file: Cardiac Imaging with MRI, CT and Nuclear Techniques, British Heart Foundation. January 2010; 13. When patients are monitored by BIOTRONIK Home Monitoring. See ProMRI® manual for all details;14. Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590; 15. Varma N et al. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up: The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) Trial. Circulation, 2010;122: 325 – 332; 16. Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590; (3.4% in the Home Monitoring Group vs. 8.7% in the control group); 17. Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679; 18. Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679; 19. Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590; 20. Vs. 55% - Crossley G H, Boyle A, Vitense H, et al. The CONNEXT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial. JACC. 2011; 57(10):1181-1189 [for bar chart comparison only]; 21. Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679; 22. Performance analysis. Data on file, 2018; 23. Performance analysis. Data on file, 2018; 24. Martin DO et al. Heart Rhythm 2012;9(11):1807-1814; 25. van Gelder BM, Bracke FA, Meijer A, Pijls NH. The hemodynamic effect of intrinsic conduction during left ventricular pacing as compared to biventricular pacing. J Am Coll Cardiol 2005;46:2305–2310; 26. Acticor/Rivacor HF-T QP, 10.8 years @ 60 ppm; RA 15%, RV 0%, LV 100% pacing, RA/RV/LV @ 2.5 V/0.4 ms; 500 Ohms, 2 max. energy shocks/year. Data on file; 27. Post-Market observation; interim-analysis, December 21, 2018. Data on file; 28. QP models only; 29. Post-Market observation; interim-analysis, December 21, 2018. Data on file; 30. Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590; (3.4% in the Home Monitoring Group vs. 8.7% in the control group).