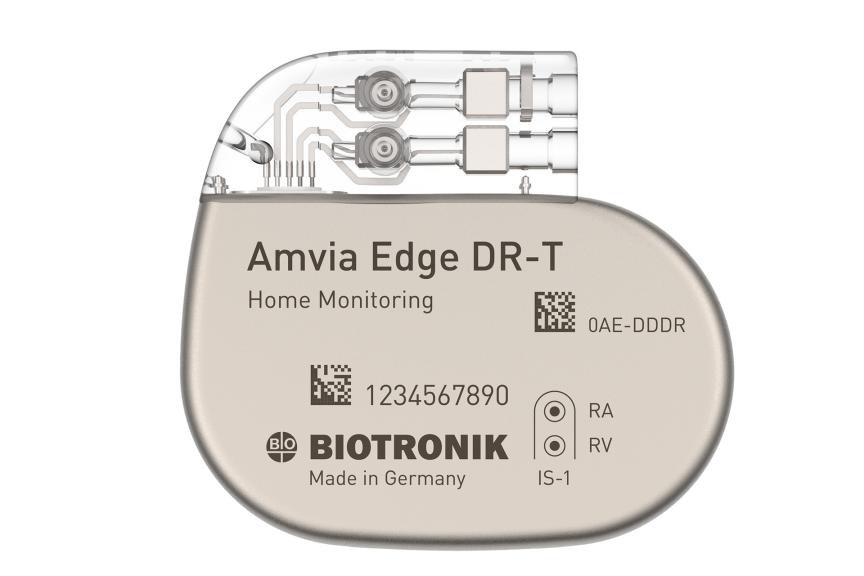
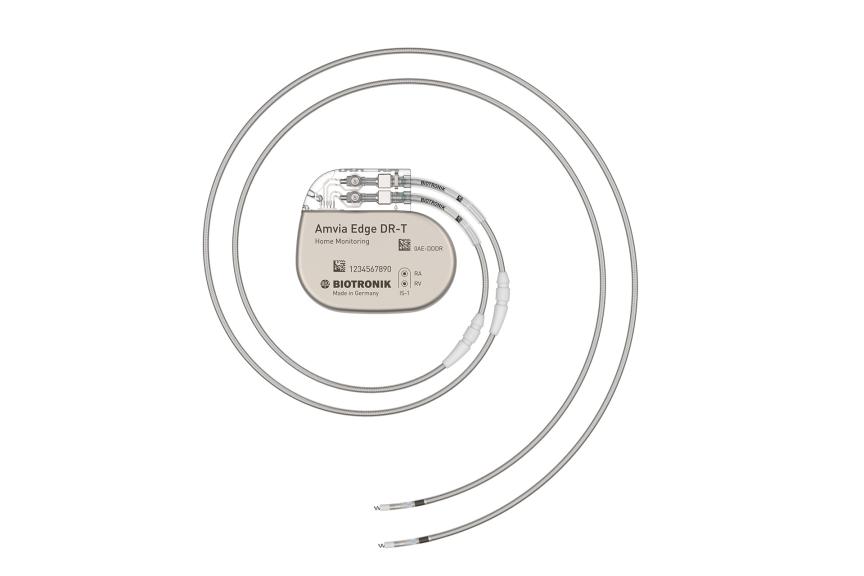
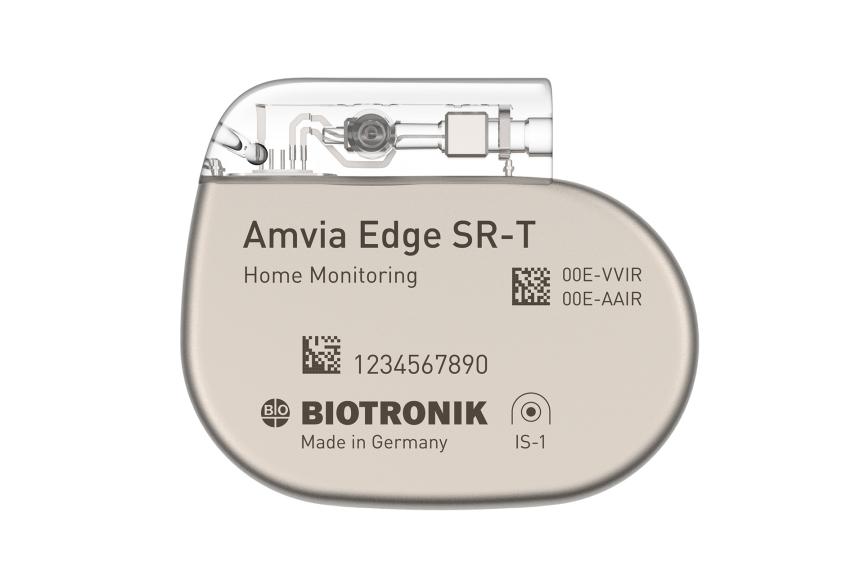
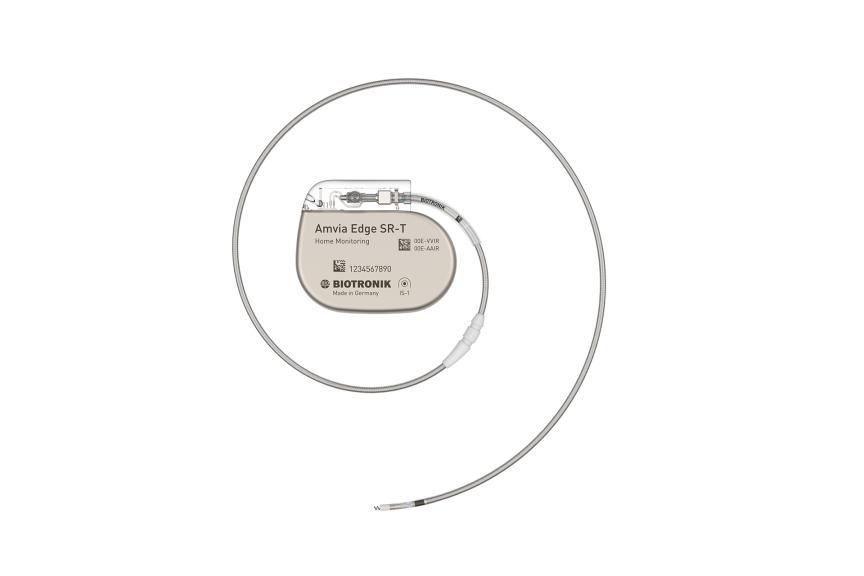
Amvia Edge DR-T/SR-T
The Amvia Edge pacing system gives you choices. Take advantage of a broader range of treatment options, including physiologic pacing and tools to address development of atrial fibrillation (AF).
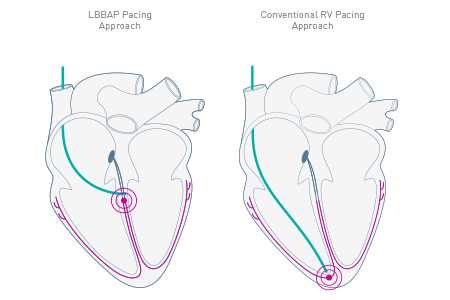
As the first pacemaker to be approved for a left bundle branch area pacing approach (LBBAP)1, Amvia Edge fosters natural myocardial contraction patterns in your patients. It provides physiologic heart rates with Closed Loop Stimulation (CLS) and aims to maintain sinus rhythm with Atrial ATP (aATP).
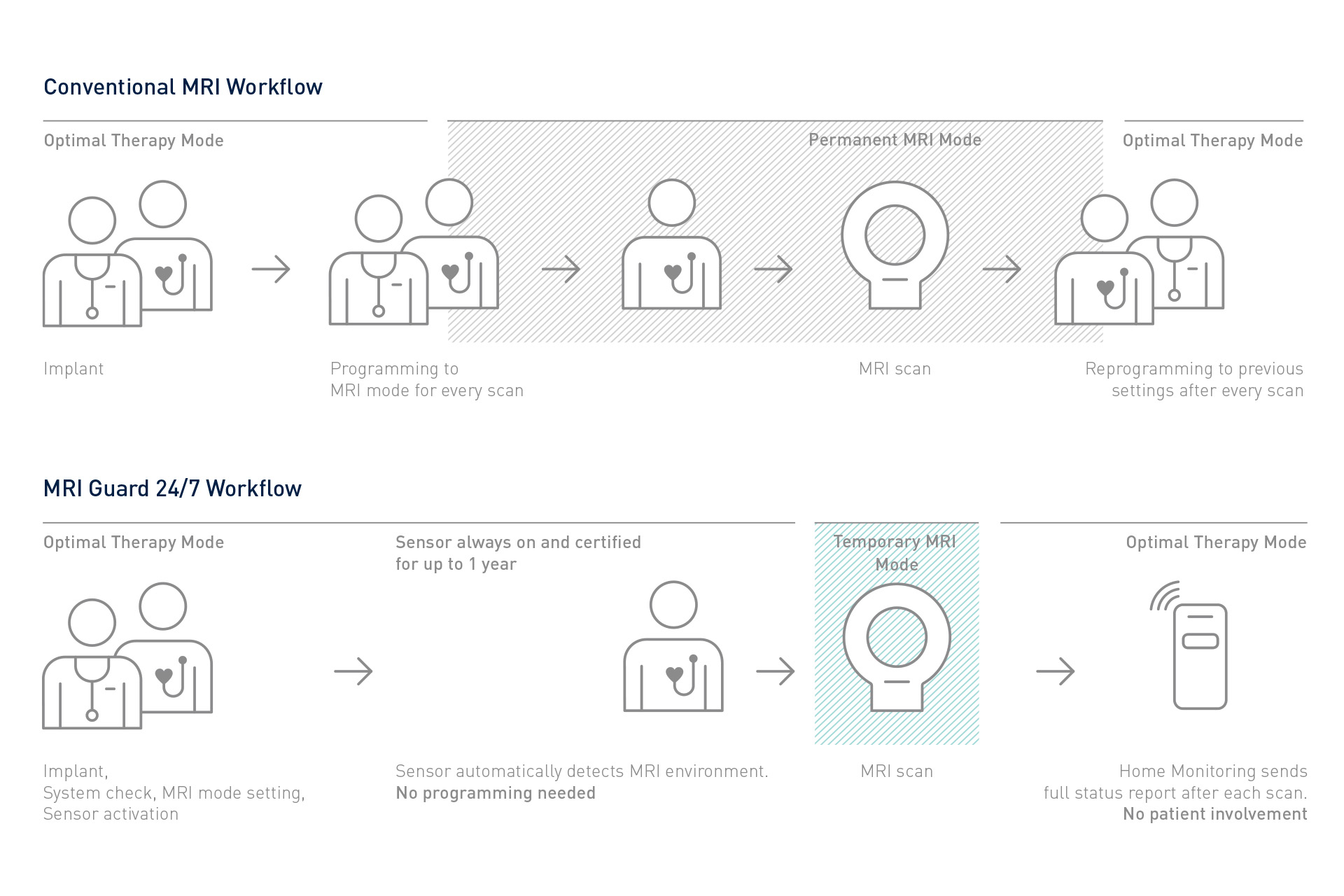
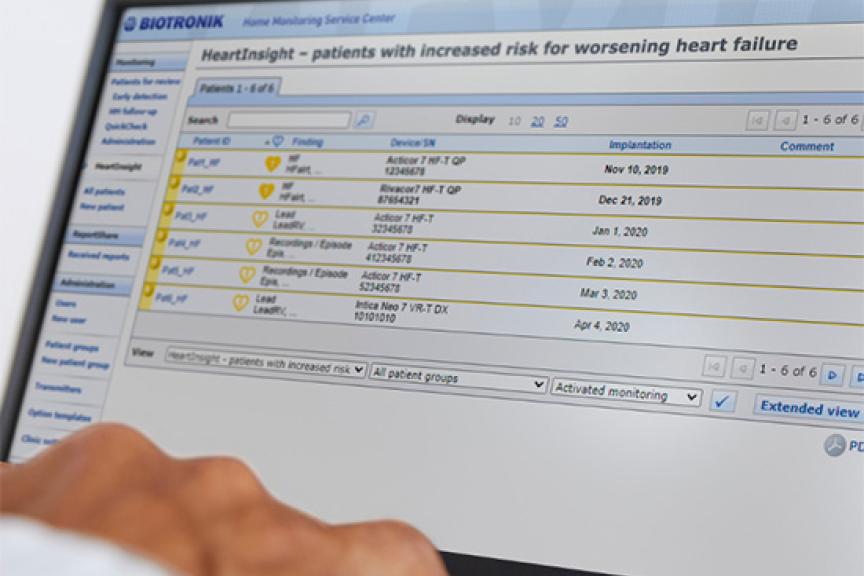
At the same time, Amvia Edge automates many routine tasks during implantation, in-office follow-ups, remote monitoring, and MRI workflows. Deliver unique physiologic pacing to your patients, enhance therapy, and free up time in daily workflows – so that more patients may benefit from more personalized care.
Product Highlights
Achieve Physiologic Pacing
Amvia Edge is the first pacemaker approved for left bundle branch area pacing (LBBAP).1 Moreover, Amvia Edge's unique Closed Loop Stimulation (CLS) sensor technology follows the autonomic nervous system and adapts automatically2 to mimic the natural intrinsic regulation.3
Manage Atrial Arrhythmias
Amvia Edge aims to help patients maintain physiologic heart rates over time. It combines in-office and remote atrial monitoring capabilities with the arrhythmia management tools of Atrial ATP (aATP) to actively address the development of atrial fibrillation (AF) early on.14
Simplify Care Pathways
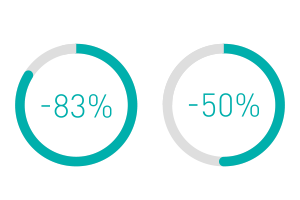
Amvia Edge includes a variety of solutions that streamline workflows throughout the patient journey, from implantation and follow-ups to remote monitoring and outstanding MRI access.
Media
Downloads & Related Links
Contact Us
References
1) BIOTRONIK Amvia Edge technical manual, Medtronic Azure XT DR MRI SureScan™ manual; Boston Scientific Accolade MRI™ technical manual; Abbott Assurity MRI™ user’s manual; MicroPort Alizea™ implant manual. 2) Lindovska M, Kameník L, Pollock B, et al. Clinical observations with Closed Loop Stimulation pacemakers in a large patient cohort: the CYLOS routine documentation registry (RECORD). Europace. 2012; 14: 1587–1595. 3) Santini M, Ricci R, Pignalberi C, et al. Effect of autonomic stressors on rate control in pacemakers using ventricular impedance signal. Pacing Clin Electrophysiol. 2004; 27: 24-32. 4) Sharma PS, Patel NR, Ravi V, et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. 2022; 19(1): 3-11. 5) De Pooter J, Ozpak E, Calle S, et al. Initial experience of left bundle branch area pacing using stylet-driven pacing leads: A multicenter study. J Cardiovasc Electrophysiol. 2022; 33(7): 1540-1549. Disclaimer: This material only summarizes the investigational use of tools for conduction system pacing (CSP) by De Pooter et al. in their clinical study. Please note that the Solia S lead is not approved for CSP. BIOTRONIK CSP tools are not currently approved for conduction system pacing in the United States. Content not intended for US healthcare providers. 6) Menezes AS, Daher MT, Nascente CM, Moreira HG, Moreira TAC, and Campos RN. Correlation among Closed Loop Stimulation, cardiopulmonary capacity, and quality of life PBMR. 2003; 8(2): 119-124. 7) Pavri BB and Russel S. An impedance sensor is superior to an accelerometer for chronotropically incompetent patients with sinus node dysfunction: results of a pilot study with a dual sensor pacemaker. Circulation. 2006; 114: II_749. 8) Coenen M, Malinowski K, Spitzer W, et al. Closed Loop Stimulation and accelerometer based rate adaptation: results of the PROVIDE study, Europace. 2008; 10: 327-333. 10) Malinowski K. Interindividual comparison of different sensor principles for rate adaptive pacing PACE. 1998; 21(PT II): 2209-2213. 10) Abi-Samra FM, Singh N, Rosin BL, DwyerJV, and Miller C. Europace. 2013; 15: 849-856. 11) Puglisi A, Favale S, Scipione P, et al. Overdrive versus conventional closed-loop rate modulation pacing in the prevention of atrial tachyarrhythmias in brady-tachy syndrome: on behalf of the Burden II study group. Pacing Clin Electrophysiol. 2008; 11: 1443-55. 12) Ikeda S, Nogami A, Inoue K, et al. Closed‐loop stimulation as a physiological rate‐modulated pacing approach based on intracardiac impedance to lower the atrial tachyarrhythmia burden in patients with sinus node dysfunction and atrial fibrillation. J Cardiovasc Electrophysiol. 2020; 31: 1187-1194. 13) Coenen M, Malinowski K, Spitz W, et al. Closed loop stimulation and accelerometer-based rate adaptation: results of the PROVIDE study. Europace. 2008; 10: 327-333. 14) Mabo P, Victor F, Bazin P, et al. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur Heart J. 2012; 33(9): 1105-1111. 15) Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008; 1(1): 62-73. 16) Data on file. 17) Varma N, Epstein AE, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010; 122(4): 325-332. 18) Mullane S, Michaelis K, Henrickson C, et al. Utilization and programming of an automatic MRI recognition feature for cardiac rhythm management devices. Heart Rhythm O2. 2021; 2: 132–137. 19). Data on file. 20) Siddamsetti S, Shinn A, Gautam S. Remote programming of cardiac implantable electronic devices: a novel approach to program cardiac devices for magnetic resonance imaging. J Cardiovasc Electrophysiol. 2022;33(5):1005–1009. 21) Watanabe E, Yamazaki F, Goto T, et al. Remote management of pacemaker patients with biennial in-clinic evaluation: continuous Home Monitoring in the Japanese At-Home study: A randomized clinical trial. Circ Arrhythm Electrophysiol. 2020 May;13(5):e007734. doi: 10.1161/CIRCEP.119.007734. 22) Garcia-Fernández FJ, Asensi JO, Romero R, et al. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: a long-term randomized trial (RM-ALONE). Eur Heart J. 2019; 40(23): 1837–1846. 23) Ricci RP, Morichelli L, Quarta L, et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring, Europace. 2010; 12(5): 674-679. 24) Data on file. 25) Data on file. 26) Amvia Edge SR-T 10 years; Limited Warranty for BIOTRONIK Cardiac Implantable Electronic Devices; Medtronic Limited Warranty Summary; Boston Scientific Limited Warranty Information and Forms; Abbott CRM Warranty Procedures Reference Manual; MicroPort Alizea DR™/Alizea SR ™ Implant Manual. 27) Amvia Edge DR-T 8 years; Limited Warranty for BIOTRONIK Cardiac Implantable Electronic Devices; Medtronic Limited Warranty Summary; Boston Scientific Limited Warranty Information and Forms; Abbott CRM Warranty Procedures Reference Manual; MicroPort Alizea DR™/Alizea SR ™ Implant Manual. 28) Amvia Edge SR-T: At 2.5 V/0.4 ms, 60 bpm, 500 Ω; pacing: 50 %, Home Monitoring: OFF, QuickCheck: OFF, RF-Telemetry: OFF. 29) Amvia Edge DR-T: A: 2.5V/0.4ms, 60 bpm, 500 Ohm, pacing: 50%, RV: 2.5V/0.4ms, 60 bpm, 500 Ohm, pacing: 5%, Home Monitoring OFF, QuickCheck: OFF, RF-Telemetry: OFF, Vp Suppression: ON.