General Information
Design Innovations and Testing on Safety
To receive approval for MRI usage, a new set of requirements were added to the design and testing of the cardiac device system. The cardiac devices and the leads are tested individually and as a combined system, as approval is only given for the specific combinations of lead(s) and devices.
Examples of additional requirements are:
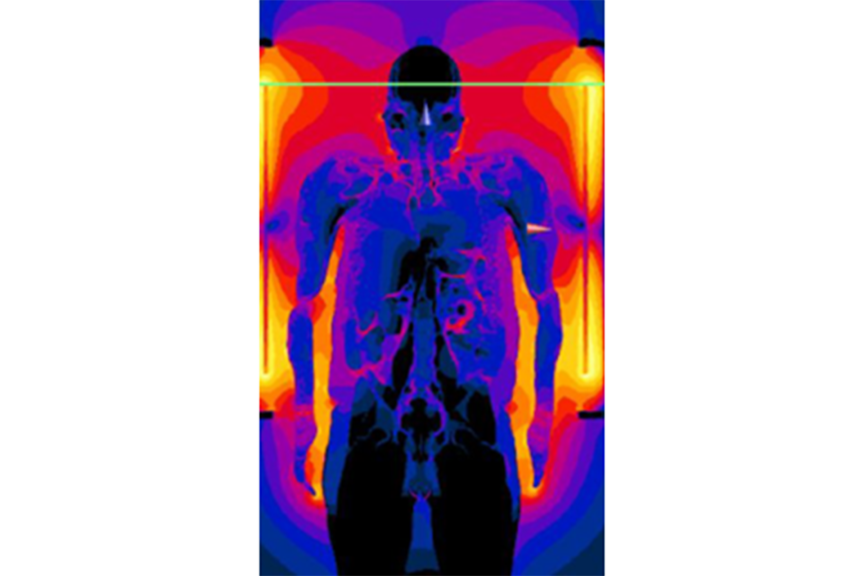
- No significant change in temperature of the lead(s) in the MRI environment. The leads can act as an antenna and dissipate the energy of the radio frequency field as temperature rise to the tip of the lead.
- Ensure optimal therapy through specific MRI modes of the devices. The MR environment may lead to inadequate sensing of the heart rhythm or unintentional pacing.
- Withstand electromagnetic interference. The strong static, gradient and RF fields present in the MR environment may affect the electrical operation of the device or its software.
Approved for MRI
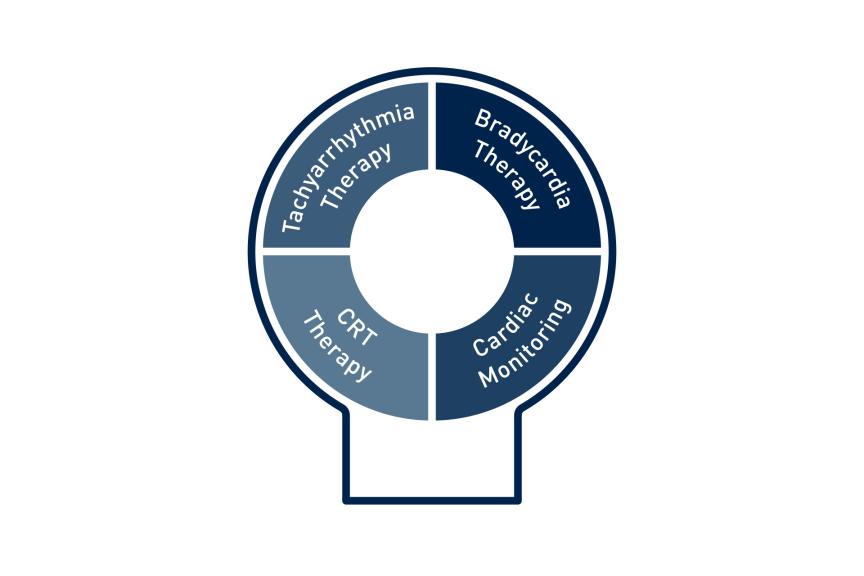
Our efforts in design and the extensive testing of the ProMRI technology in our products have resulted in MR conditional devices in all areas of arrhythmia management:
- MR conditional cardiac monitors, pacemakers, ICDs, CRT-P and CRT-D systems and their leads
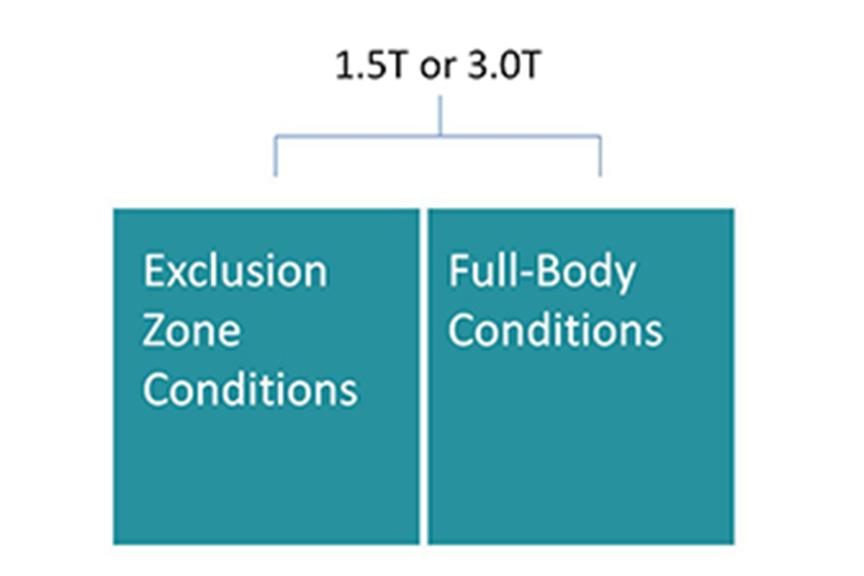
- Cardiac devices approved for 1.5 Tesla and 3.0 Tesla MRI scanners
- More than 1.000.000 sold products MRI approved worldwide
To minimize barriers for device patients to undergo the MRI scanning they need, we continue our efforts to have our MR conditional systems approved for 1.5 T and 3.0 T MRI scanners, allowing full-body MRI scanning and streamlining the scanning procedure.
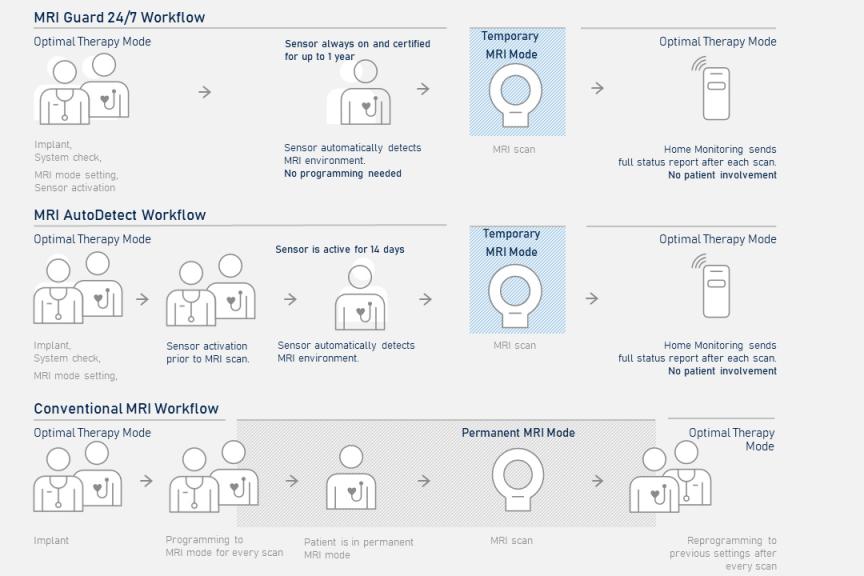
The latest innovation in this field is MRI AutoDetect. Cardiac devices with this feature have a new built-in sensor detecting the MR environment and automatically adapting the therapy mode during the MRI scanning. As a result, the workflow of device programming is improved and the scanning procedure for cardiac device patients is simplified
MRI Access: A Growing Need
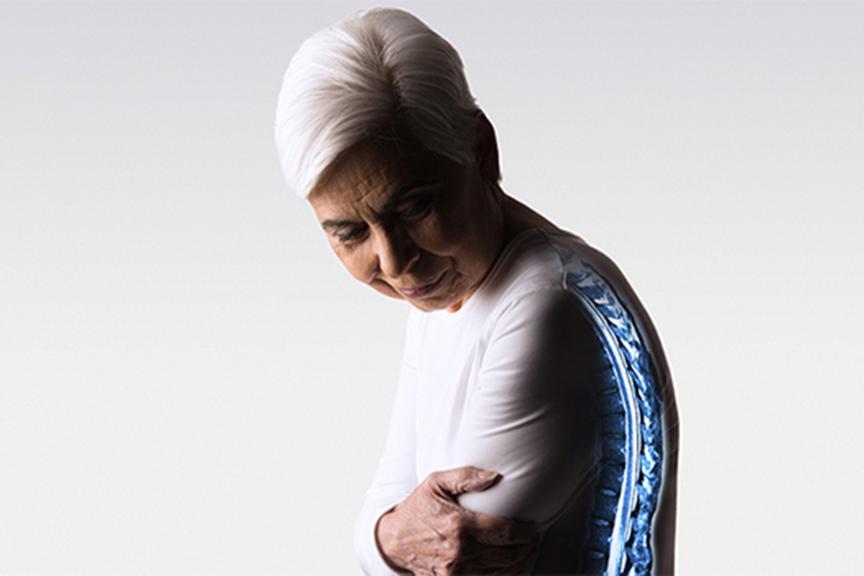
MR imaging is the preferred diagnostic modality for many soft tissue, sometimes life threatening conditions. As many patients with a cardiac device also have other comorbidities, up to 75% of these patients will need an MRI in their lifetime1.
In 2012, the overall number of MRI scans in the US reached 30.2 million procedures2. MRI volumes will continue to be driven by growth in brain, musculoskeletal, and spine applications, in addition to newer procedures like cardiac MRI.
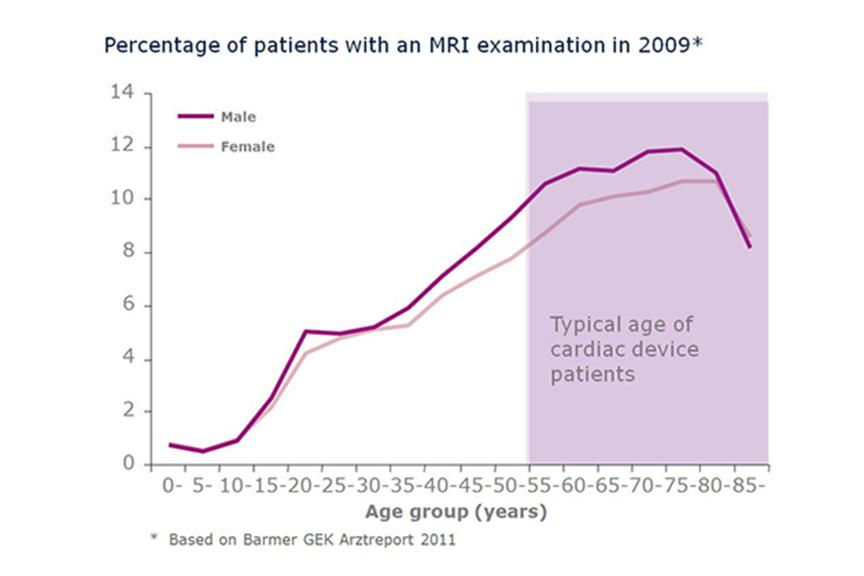
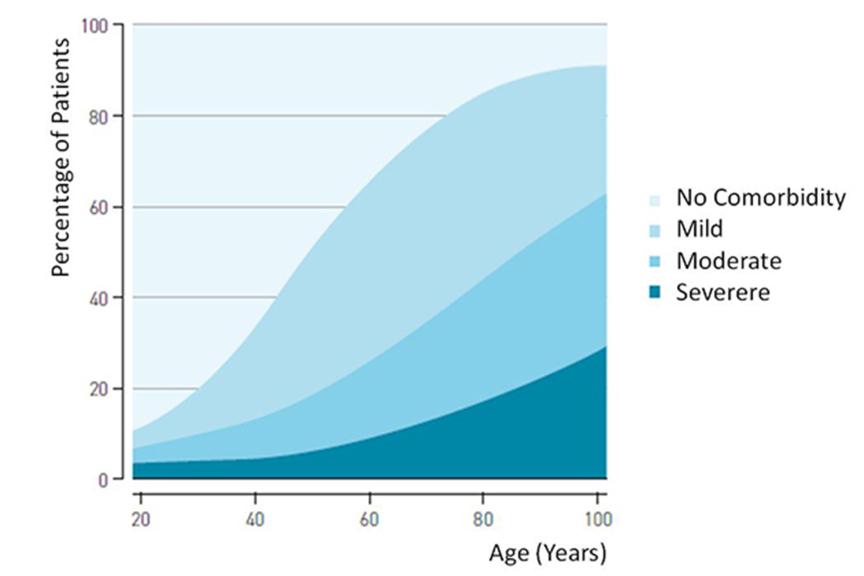
The need for MRI scanning is highest in the age group between 55-80 years, patients in this age group are also more likely to receive a cardiac device. As the demographics in many countries show a population growth of elderly people, an increase in demand of MRI scans with patients implanted with cardiac devices is expected as well.
The growing number of patients with an implanted cardiac device and the increased use of MRI to diagnose diseases like cancer or stroke will collide and result in more and more patients missing out on MRI scans. For the US in 2004 alone, it was estimated that 200,000 device patients missed out on MRI scans3. To counteract this problem, BIOTRONIK continuously strives to eliminate these barriers by introducing MR conditional pacemakers, leads and other cardiac devices.