BIOSOLVE-II
NCT01960504
First in man study of the DREAMS 2nd generation drug-eluting absorbable metal scaffold
Conclusions
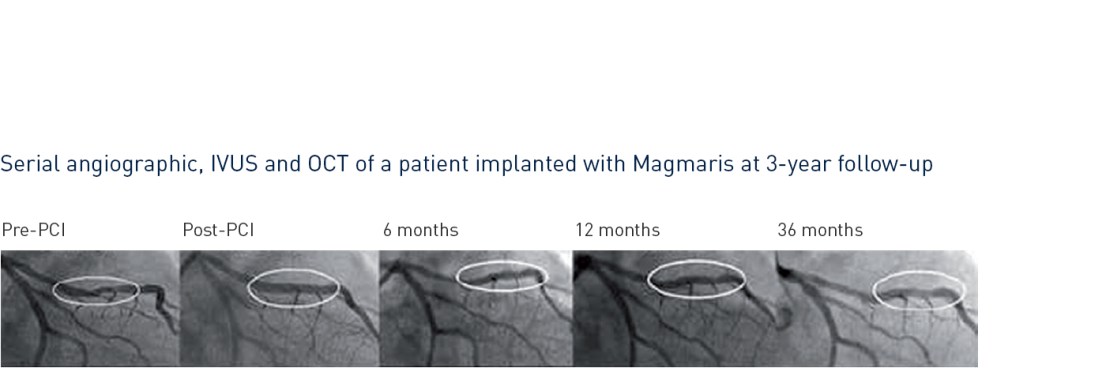
- Magmaris demonstrated a favorable safety and performance until 24-month follow-up
- The rate of definite/probable scaffold thrombosis remained at 0% at 24 months
Study Design
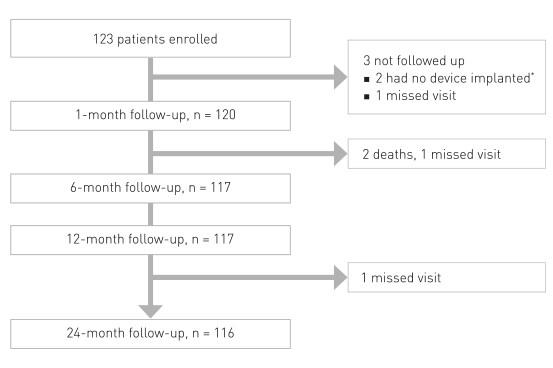
Prospective, multi-center, first-in-man trial to evaluate the safety and performance of Magmaris in 123 patients with a maximum of two de novo lesions in two separate coronary arteries
Principal investigator:
Prof. Michael Haude, Neuss, Germany
* Two patients who did not receive an implant were used for calculation of device and procedural success only.
Primary endpoint:
In-segment Late Lumen Loss (LLL) at 6-month follow-up
Secondary endpoints (selected):
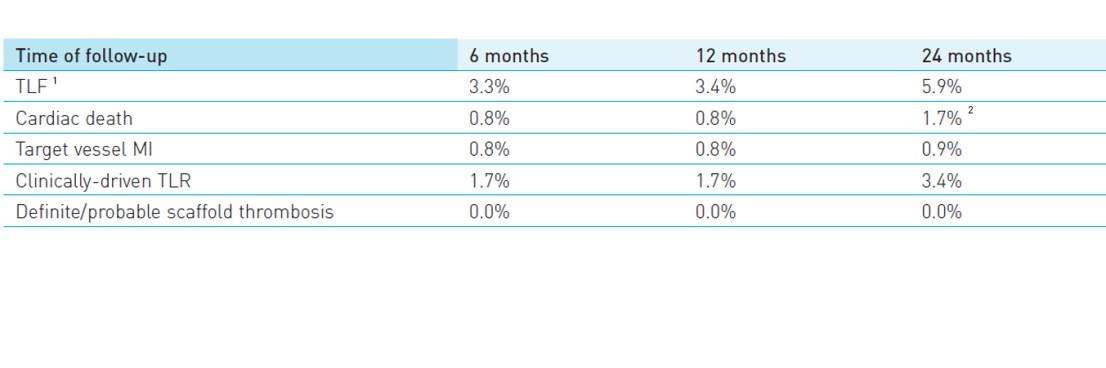
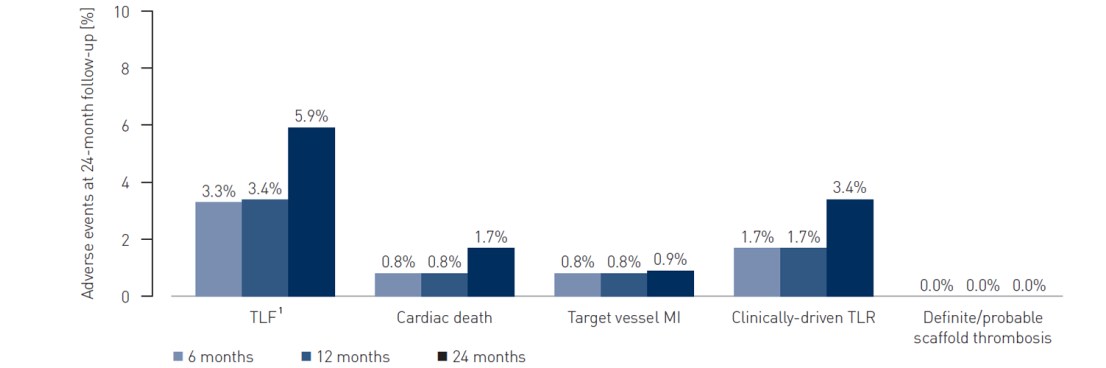
- TLF defined as a composite of cardiac death, target-vessel myocardial infarction and clinically-driven target lesion revascularization (cd-TLR) at 24 months
- Definite/probable scaffold thrombosis at 24 months
Clinical Results
Downloads


Vascular Intervention
Clinical StudyFirst in man trial with DREAMS (Drug Eluting Absorbable Metal Scaffold)
Disclaimer
Magmaris is not available in the US.
© BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.
1 TLF defined as a composite of cardiac death, target-vessel MI and cd-TLR
2 Two deaths of unknown cause were adjudicated as cardiac deaths
