Product Details
Easy Release
Relieves friction of introducer valve on the retractable shaft during stent deployment for a smoother action.

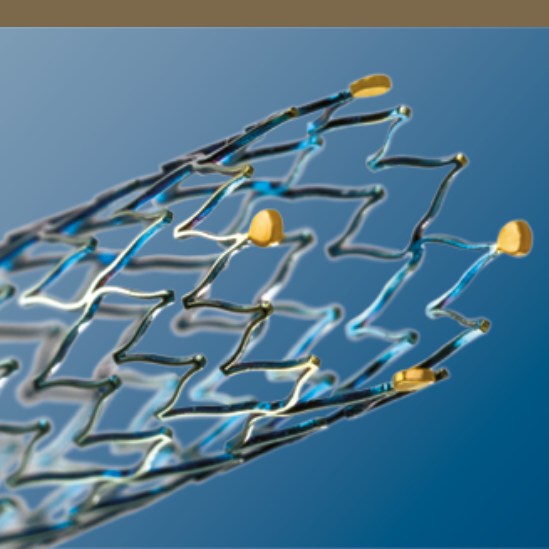
Segmented Stent Design for Iliac Artery
- Peak-to-valley design and S-articulating connecting bars provide multi-directional flexibility and avoid fish-scaling in tortuous arteries.
- Segmented design and strut thickness designed to provide sufficient chronic outward force.

Enhanced Visibility
Four gold markers at each end of the stent enhance visibility.
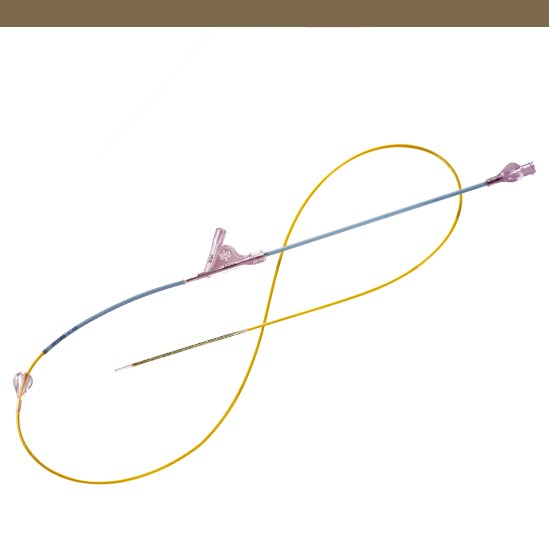
Pull-Back Delivery System
Enables simple stent deployment.
Safety Tab
Avoids accidental stent deployment.

Improved Stent Surface Biocompatibility*
Excellent results with proBIO coated stents. Effective and
reliable barrier to nickel and other metal ion diffusion.
6 F Introducer Compatibility
6 F distal shaft with 5.2 F proximal shaft for contrast injection with device positioned inside the introducer and across lesion.
BIOFLEX-I
- Prospective, international, multi-center, investigational device exemption trial evaluating BIOTRONIK Astron nitinol self-expanding stent for iliac arteries, conducted at 30 centers in the US, Canada and Europe.
- Number of patients (n): 161
- Primary endpoint: composite rate of procedure- or stent-related MAEs at 12 months, post-index procedure (30-day mortality, 12-month clinically-driven TLR and index-limb amputation)
Technical Data
| Astron Stent | |
|---|---|
| Catheter type | OTW |
| Recommended guide wire | 0.035" |
| Stent material | Nitinol |
| Strut thickness | 225 μm (ø 10 mm = 230 μm) |
| Stent coating | proBIO (amorphous silicone carbide) |
| Stent markers | 4 gold markers each end |
| Sizes | ø 7 - 10 mm; L: 30 - 80 mm |
| Proximal shaft | 5.2 F, hydrophobic coating |
| Usable length | 70 and 120 cm |