
Ability to remotely monitor atrial high-rate episodes using a single-chamber implantable cardioverter-defibrillator with a floating atrial sensing dipole
Hindricks G, Bollmann A et al., EUROPACE 2023
https://doi.org/10.1093/europace/euad061
Largest Trial on DX Technology in Single-Chamber ICD Patients
The 2020 ESC Guidelines for Diagnosis and Management of Atrial Fibrillation (AF)2 recommend remote monitoring (RM) of device-detected atrial high rate episodes (AHRE) and propose options for managing AHRE along pre-specified strata (6min…<1h, 1h…<24h, ≥24h)3 . DX Technology is unique in that it offers true atrial sensing in single-chamber ICD systems. This analysis aimed to assess the DX-ICD system’s capability to follow this recommendation, and to evaluate findings on AHRE detection and progression regarding their clinical implications based on real-life MATRIX registry data. MATRIX is the largest trial involving DX-ICD systems to date, enrolling 2,054 patients in 119 centers worldwide.
The study found that the DX-ICD system demonstrates a high detection accuracy for AHRE throughout 2 years of follow-up. Combined with the high transmission rate of BIOTRONIK Home Monitoring (HM), this allows reliable, guideline-recommended screening for subclinical AF and monitoring of AHRE duration progression.
Furthermore, the clinical relevance of new-onset AHRE detection and burden monitoring was underlined by MATRIX results, as new-onset AHRE was not rare, and progression shown to be common in these patients. In fact, the majority of patients with new-onset AHRE (69.5%) presented with AHRE of longer duration (i.e., 1 hour or more) during follow-up. More importantly, 80% of patients with new-onset AHRE had a substantial risk of thromboembolic events (i.e. a high CHA2DS2-VASc score) - and in most of these cases (69.5%), patients were not on oral anticoagulation (OAC) at baseline.
Read more about the study design, key results, and clinical relevance below.
Study Design
- International, multicenter registry including 119 sites in 24 countries
- 2,054 DX-ICD patients with standard single-chamber ICD indication enrolled
- Follow-up 24 months, according to the centers’ routine follow-up scheme (i.e., in a real-life setting)
- BIOTRONIK (Home Monitoring recommended)
Two objectives:
- To evaluate the ability of a DX-ICD system to remotely monitor AHRE and progression of arrhythmia duration (i.e., to implement the guideline-recommended remote monitoring of subclinical AF), and
- To analyze the clinical implications of findings on AHRE detection and progression.
Key Result 1 Highly Accurate AHRE Detection
Adjudication of Device-Detected AHRE Shows High Positive Predictive Value
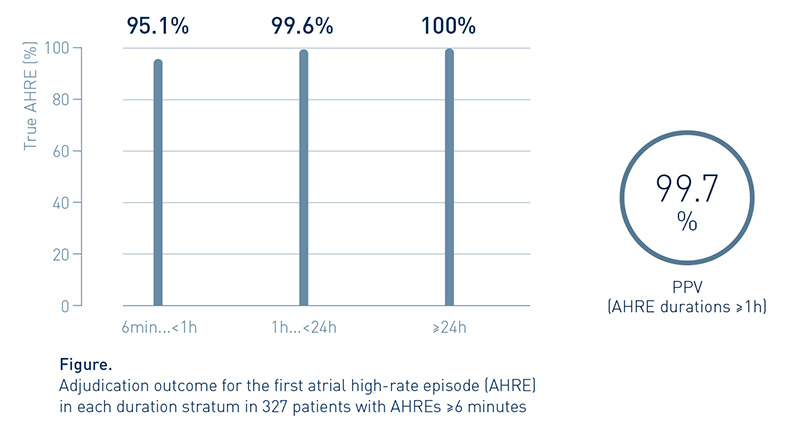
Analyzing the detection accuracy of DX-ICD systems for different AHRE durations, the first occurring AHRE per duration stratum was adjudicated, revealing a positive predictive value (PPV) of >99.7% for AHRE durations ≥1h.
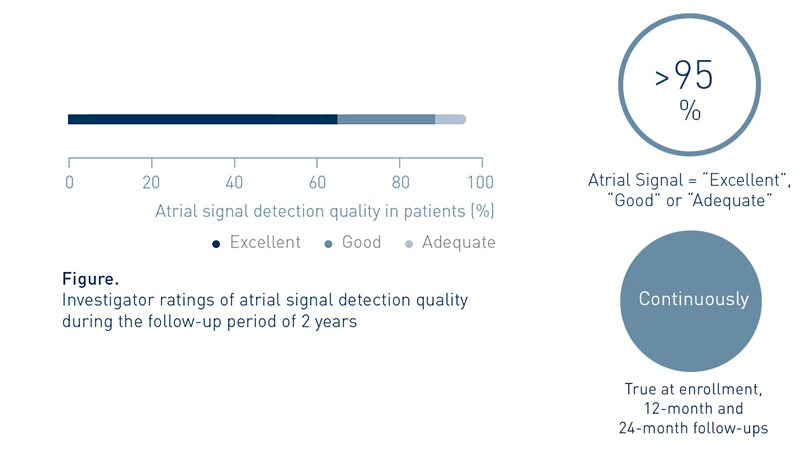
Investigators Value the System’s Atrial Signal Detection Quality
Key Result 2 Reliable Monitoring
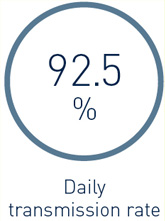
DX-ICD systems including Home Monitoring (HM) delivered a high real-life transmission performance: In patients with active HM (n=1,841), the median daily transmission rate (i.e., % of days with transmissions) was 92.5%.
Capability to Implement Guideline-Recommended Subclinical AF Monitoring
The system's high transmission rate, combined with its high AHRE detection accuracy, allows for reliable, guideline -recommended remote monitoring of subclinical AF.
Key Result 3 New-Onset AHRE Not Rare, Progression and High Stroke Risk Common
New-Onset AHRE Is Not Rare
In patients with no AF history (n = 1,451), new-onset AHRE occurred in 119 patients (8.2%) during the 2 years of follow-up.
Progression Is Common
Progression to a stratum of longer duration was rather frequent (31.1%)
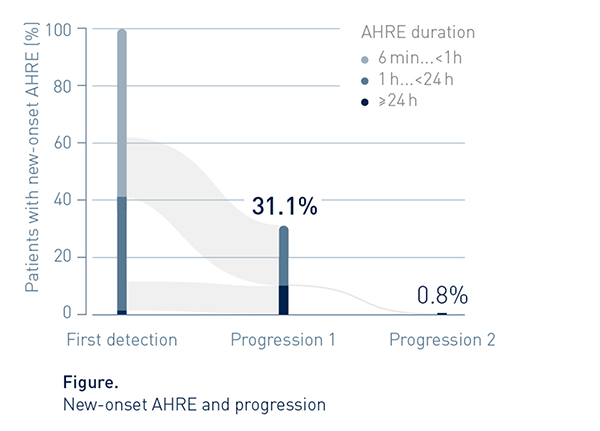
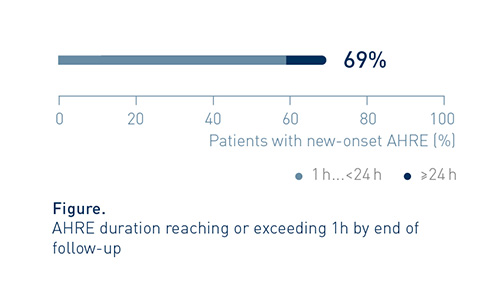
Episode Duration Often Exceeded 1h
By end of follow-up, AHRE duration reached or exceeded 1h in approx. 7 out of 10 patients (82/119, 69%) with new onset AHRE.
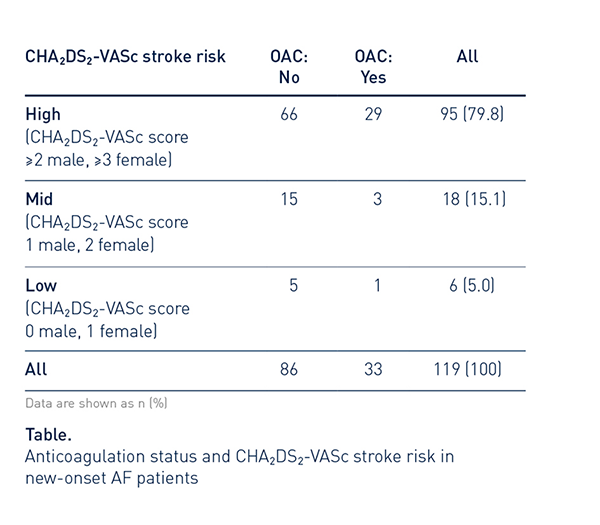
Many New-Onset AHRE Patients Were at High Risk of Thromboembolic Events
79.8% of patients with new-onset AHRE had a high CHA2DS2-VASc stroke risk (95/119).
Most of these were not on OAC therapy at baseline (66/95, 69.5%).
Clinical Relevance
- MATRIX is largest clinical evaluation of DX Technology to date
- In this unselected, real-life setting, the findings confirm that DX Technology, combined with Home Monitoring, allows for reliable, guideline-recommended remote monitoring of subclinical AF
- The clinical relevance of AHRE monitoring is underlined by the fact that patients with new-onset AHRE often experience AHRE progression and are at substantial risk of thromboembolic events, while most of them are not on OAC.
| Study Objective | To explore the impact of enhanced features and capabilities of the BIOTRONIK DX system on detection and management of AF in an unselected real-life setting. |
|---|---|
| Clinical Sites |
|
| Sample Size |
|
| Main Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Devices |
|
| Follow-up |
|
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|
Downloads
References
i AHRE lasting ≥ 6 minutes were included in the analysis.
"Active": at least 1 HM transmission was received during the 24 months of follow-up.
iii transmission rate = % of days with transmissions
