Magmaris®
Magmaris is the first clinically proven resorbable Magnesium scaffold.1-4 It is currently indicated for de novo lesions with a reference vessel diameter and lesion length closely matching the available Magmaris sizes.ᵃ⁵
Product Highlights
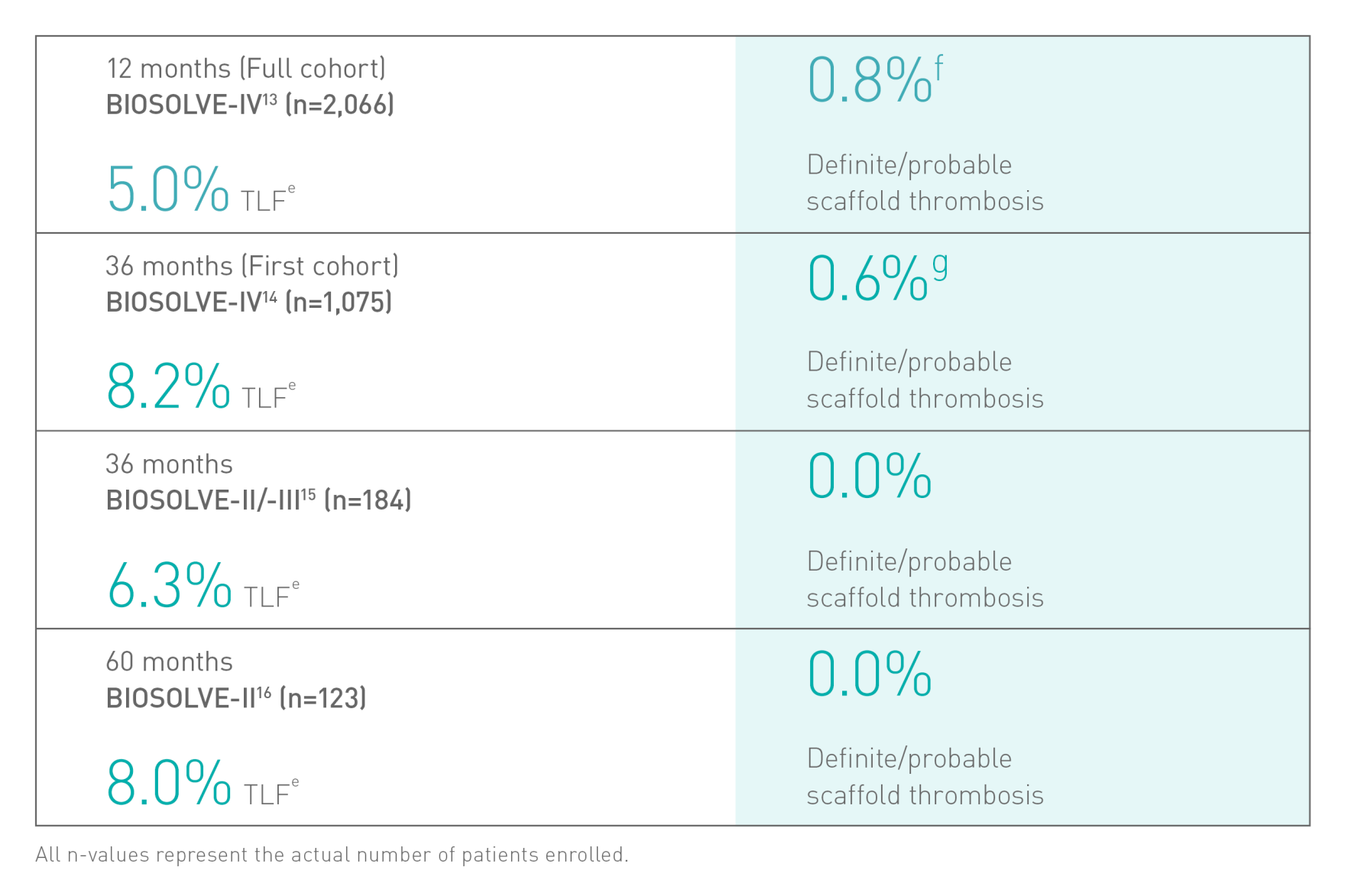
Confirmed clinical safety and efficacyb6-8
Confidence through evidence
Fast Magnesium resorption time
~95% of Magnesium resorbed at 12 months9
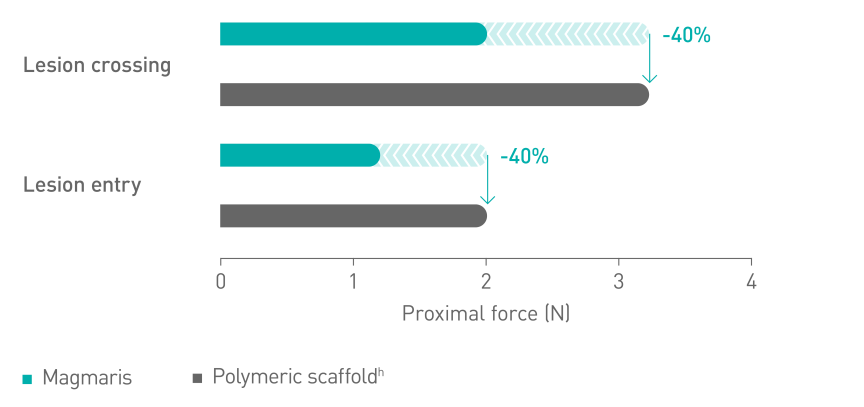
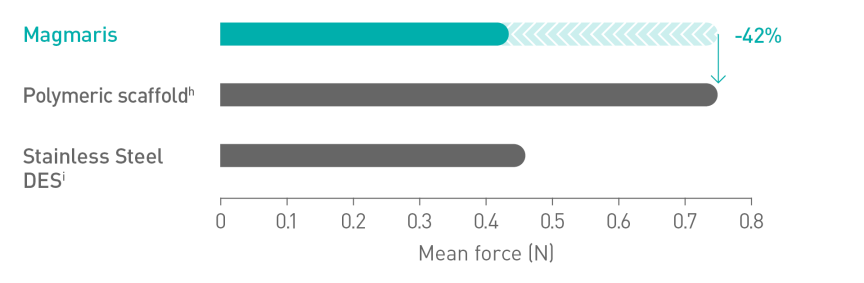
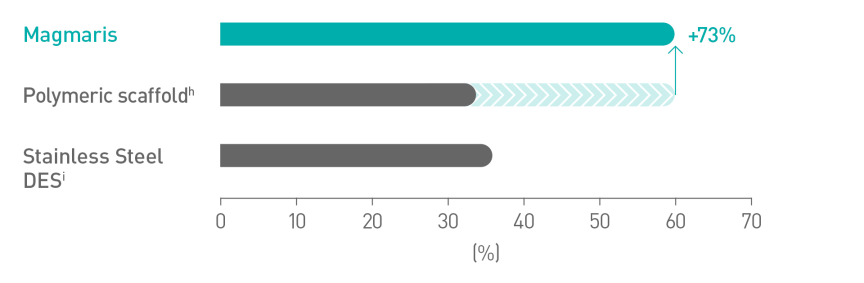
Better deliverabilityc10
Magnesium allows for a smoother scaffold surface11
Product Overview
Magmaris

Double eye radiopaque markers
Magnesium backbone
BIOlute® Coating
Technical Data
| Scaffold | |
|---|---|
| Scaffold material | Proprietary Magnesium alloy |
| Markers | Two tantalum markers at each end |
| Active coating | BIOlute® (resorbable Poly-L-Lactide (PLLA) eluting a limus drug) |
| Drug dose | 1.4 μg/mm2 |
| Strut thickness/width | 150 μm/150 μm |
| Maximum expandable diameter |
Nominal Diameter +0.6 mm |
| Delivery system | |
| Catheter type | Rapid exchange |
| Recommended guide catheter | 6F (min. I.D. 0.070”) |
| Crossing profile | 1.5 mm |
| Guide wire diameter | 0.014” |
| Usable catheter length | 140 cm |
| Balloon material | Semi-crystalline polymer |
| Coating (distal shaft) | Dual coated |
| Marker bands | Two swaged platinum-iridium markers |
| Proximal shaft diameter | 2.0F |
| Distal shaft diameter | 2.9F |
| Nominal pressure (NP) | 10 atm |
| Rate burst pressure (RBP) | 16 atm |
Compliance Chart
| Balloon diameter (mm) | |||
|---|---|---|---|
| ø 3.00 | ø 3.50 | ||
| Nominal Pressure (NP) | atm* | 10 | 10 |
| ø (mm) | 3.00 | 3.54 | |
| Rated Burst Pressure (RBP) | atm* | 16 | 16 |
| ø (mm) | 3.29 | 3.82 | |
| *1 atm = 1.013 bar | |||
Ordering Information
| Scaffold ø (mm) |
Scaffold length (mm) |
||
|---|---|---|---|
| 15 | 20 | 25 | |
| 3.00 | 412526 | 412527 | 412528 |
| 3.50 | 412529 | 412530 | 412531 |
Downloads and Related Links
Downloads
Related Links
Media
Product Animations
How can we help you?
References
a. Indication as per IFU; b. Based on BIOSOLVE-II, -II/-III and -IV, for patient populations see study details; c. compared to leading polymeric scaffold - Abbott Absorb; d. BIOSOLVE-IV 2-year follow-up for the full cohort (n=2066 patients); e.Target Lesion Failure (TLF) defined as a composite of Cardiac death, Target-Vessel Myocardial Infarction (TV-MI); emergent Coronary Artery Bypass Grafting (eCABG); Clinically-Driven Target Lesion Revascularization (CD-TLR); f. 0.4% of cases without early antiplatelet or anticoagulant interruption at post procedure; g. 0.5% scaffold thrombosis rate excluding cases with early antiplatelet or anticoagulant interruption; h. Absorb, Abbott; i. BioFreedom, Biosensors.
1. Erbel R. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007 Jun 2;369(9576):1869-1875. doi: 10.1016/S0140-6736(07)60853-8; 2. Haude M et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first in-man BIOSOLVE-I trial. Lancet. 2013 Mar 9;381(9869):836-44; 3. Wang et al. Vascular restoration therapy and bioresorbable vascular scaffold. Regenerative Biomaterials, 2014, 49–55. doi: 10.1093/rb/rbu005; 4. Haude M et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month|results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet.2016;Jan 2;387(10013):31-9. doi: 10.1016/S0140-6736(15)00447-X. Epub 2015 Oct 12; 5. Fajadet J et al._Magmaris preliminary recommendation upon commercial|launch: a consensus from the expert panel on 14 April 2016. EuroIntervention. 2016;12:828-833; 6. Torzewski J. Safety and performance of Magmaris at 24 month follow up of BIOSOLVE IV. Presented at: eEuroPCR; 2021; virtual congress. ClinicalTrials.gov: NCT02817802; 7. Haude M. Long-term clinical data of the BIOSOLVE-II study with the drug-eluting absorbable metal scaffold in the treatment of subjects with de novo lesions in native coronary arteries - BIOSOLVE-II. Presented at the: e-Course PCR, 25.June 2020, Paris, France; 8. Haude M, et al. Sustained safety and performance of the second-generation sirolimus-eluting absorbable metal scaffold: Pooled outcomes of the BIOSOLVE-II and -III trials at 3 years. Cardiovascular Revascularization Medicine. 2020. doi:10.1016/j.carrev.2020.04.006; 9. Joner M, Ruppelt P, Zumstein P, et al. Preclinical Evaluation of Degradation Kinetics and Elemental Mapping of First and Second Generation Bioresorbable Magnesium Scaffolds. EuroIntervention. 2018 Feb 20. pii: EIJ-D-17-00708. doi: 10.4244/EIJ-D-17-00708. [Epub ahead of print]; 10. Schmidt et al. In vitro performance investigation of bioresorbable scaffolds – standard tests for vascular stents and beyond. Cardiovasc Revasc Med. 2016 Sep;17(6):375-83. doi: 10.1016/j.carrev.2016.05.001. Epub 2016 May 13; 11. BIOTRONIK data on file; 12. Bennett J. Safety and Efficacy of the Resorbable Magnesium Scaffold, Magmaris in a Real-World Setting – 24-month Follow-up of the Full Cohort (2066 subjects) of the BIOSOLVE-IV Registry. Presented at: TCT, September 2022, Boston, USA. ClinicalTrials.gov: NCT02817802; 13. Bennett J. Performance and safety of the resorbable magnesium scaffold, Magmaris in a real-world setting – Primary and secondary endpoint analysis of the full cohort (2,066 subjects) of the BIOSOLVE-IV, Presented at: TCT 2021, November 2021, Orlando, USA. ClinicalTrials.gov: NCT02817802; 14. Torzewski J. Safety and performance of Magmaris at 36-months: BIOSOLVE-IV first cohort. Presented at: EuroPCR; 2022; ClinicalTrials.gov: NCT02817802; 15. Haude M, et al. Sustained safety and performance of the second-generation sirolimus-eluting absorbable metal scaffold: Pooled outcomes of the BIOSOLVE-II and -III trials at 3 years. Cardiovascular Revascularization Medicine. 2020. doi: 10.1016/j.carrev.2020.04.006; 16. Haude M, et al. Sustained safety and performance of a second-generation sirolimus-eluting absorbable metal scaffold: Long-term data of the BIOSOLVE-II first-in-man trial at 5 years. Cardiovascular Revascularization Medicine. 2021. doi: 10.1016/j.carrev.2021.07.017; 17. BIOSOLVE-II case, GER443-012. Courtesy of Prof. M. Haude, Rheinland Klinikum Neuss GmbH, Neuss, Germany 2015; 18. Torzewski J. Safety and performance of Magmaris at 48 months: BIOSOLVE-IV first cohort. Presented at: EuroPCR; 2023; ClinicalTrials.gov: NCT02817802.
BIOSOLVE-II and -IV based on Kaplan-Meier failure estimate analysis including censored observations. The pooled analysis of BIOSOLVE-II and -III based on frequency analysis. The 36-month data of BIOSOLVE-II and -III analysis reflecting a period up to 1’125 days at 3 years.
Magmaris and BIOlute are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
All other trademarks are the property of their respective owners.