Vascular Intervention
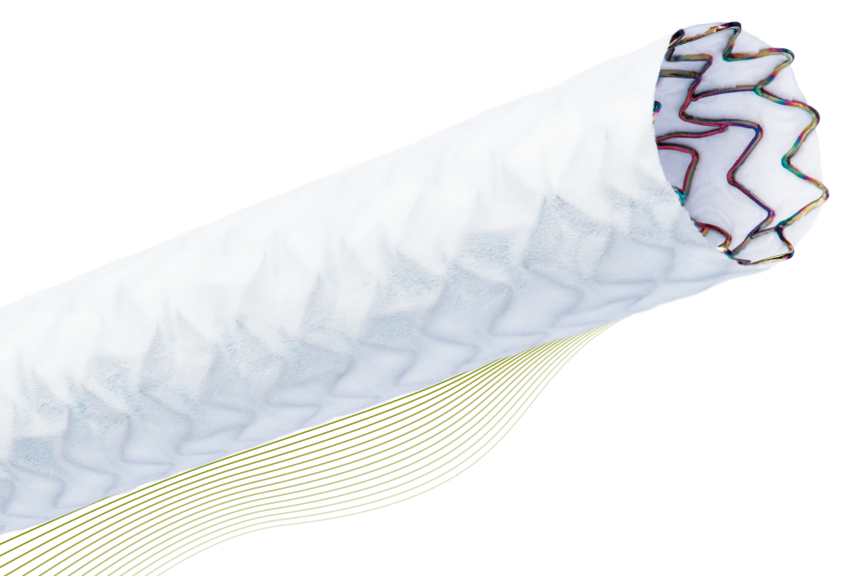
Our broad range of products work to restore blood flow to vessels of the heart (coronary) and body (peripheral) that are narrowed or blocked. We produce a full range of stent systems, balloon catheters and guide wires for patients with coronary and peripheral artery disease.
BIOTRONIK is a true innovator in the field, having introduced Pulsar, the world's first 4 F-compatible stent for treating long lesions, in 2009 and Orsiro, the industry's first hybrid drug-eluting stent, in 2011. Over the years our wide range of vascular intervention innovations include Orsiro DES, Resorbable Magnesium Scaffold, Drug Coated Balloon Catheters, Multifunctional Catheters and many more. Patient well being is our top priority and has been for 60 years.
Image