One-Year BIONETIC-I Study Results Show Safety and Effectiveness of Iliac Artery Treatment With BIOTRONIK’s Dynetic-35 Cobalt Chromium Balloon-Expandable Stent System New Data Presented at LINC 2024 Support Use of Thin-Strut Cobalt Chromium Stent in Calcified Iliac Lesions
BIOTRONIK announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC 2024.
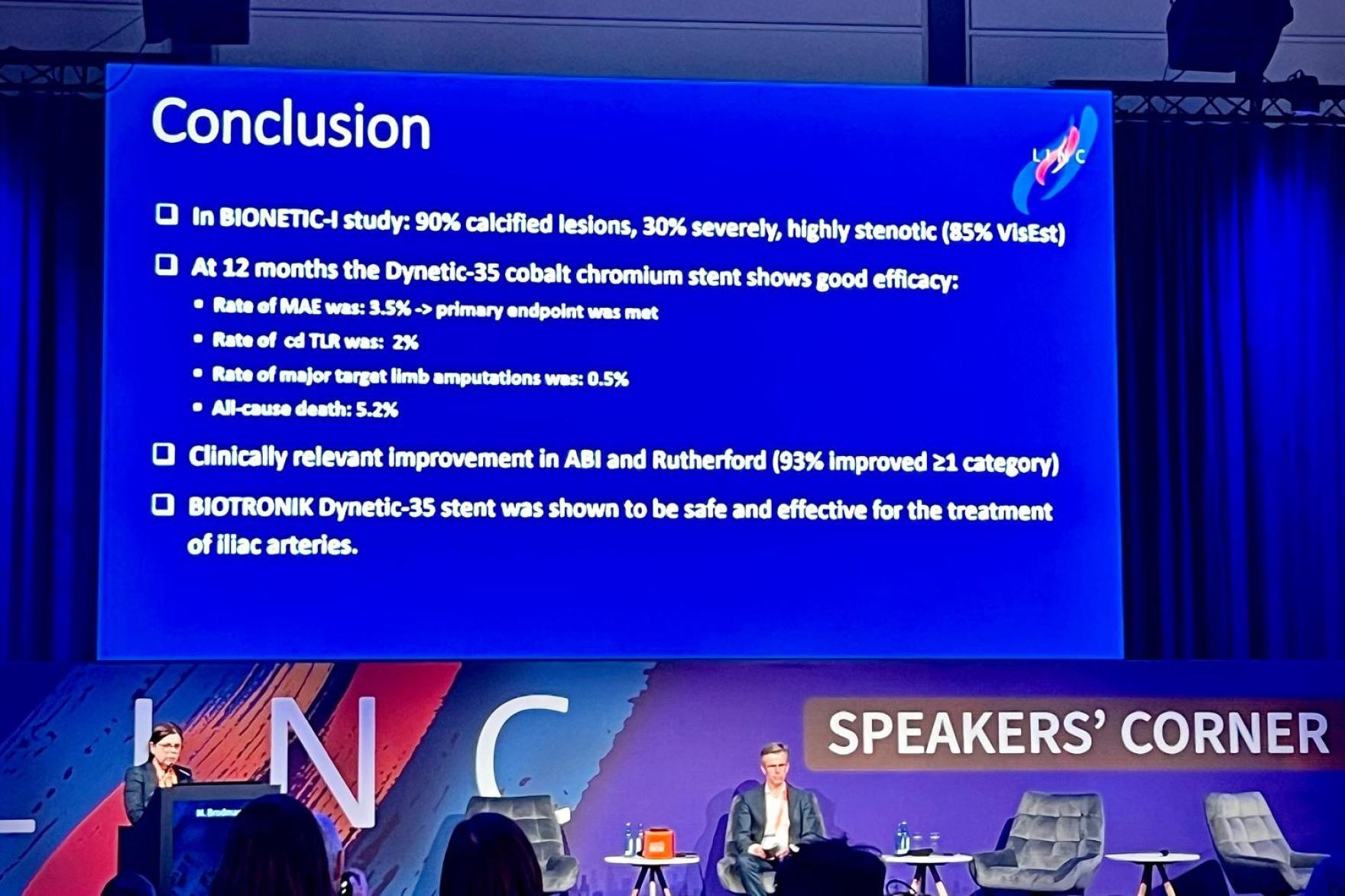
The prospective, international, multicenter single-arm observational study evaluated the treatment of de novo, restenotic or occluded iliac lesions in 160 patients with Rutherford Class 2-6 peripheral artery disease using the Dynetic®-35 cobalt chromium balloon-expandable stent system. At baseline, 12.5% of enrolled patients had critical limb ischemia, 90% had calcified lesions (30.7% severe calcification), and there was an average of 85.5% stenosis in the target lesion.
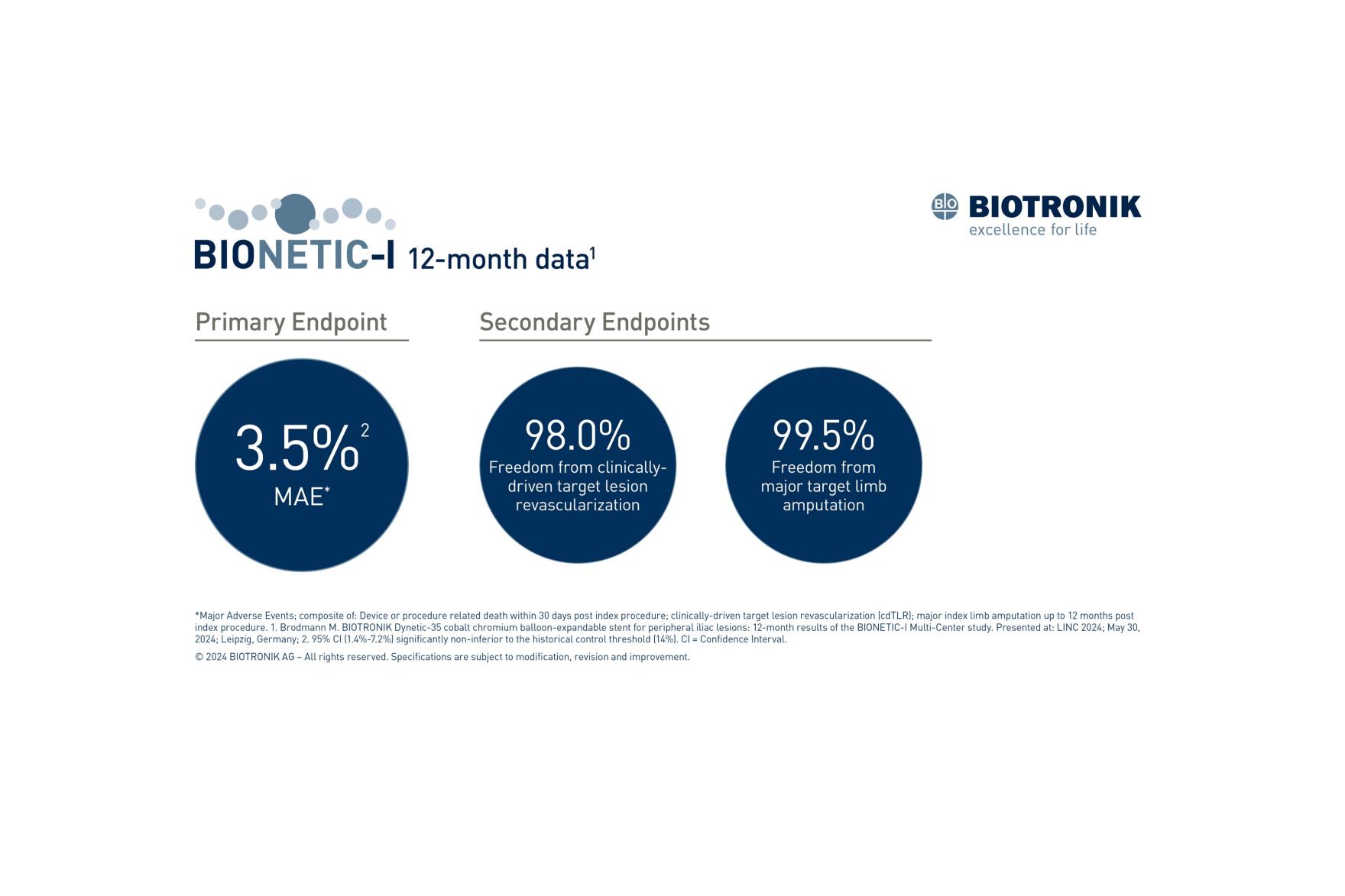
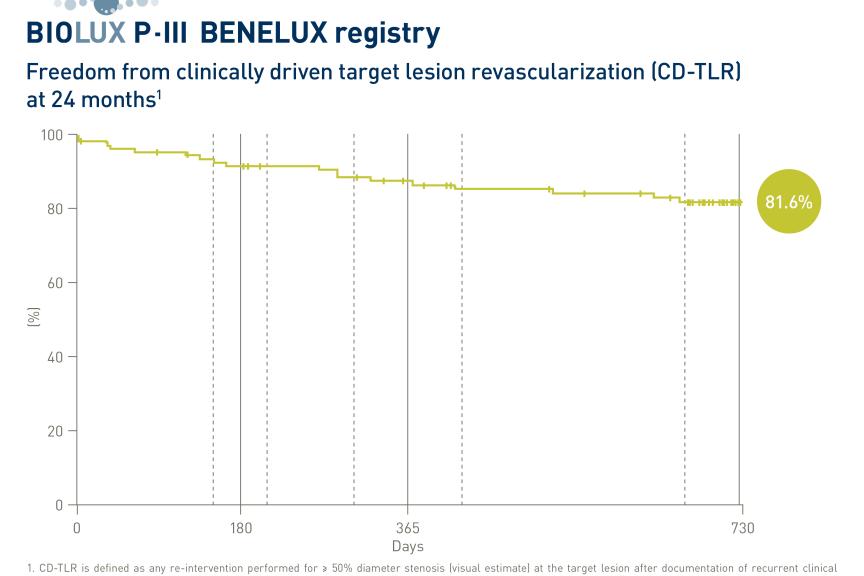
The primary endpoint, major adverse events (MAE) at 12 months, was met with a 3.5% rate of MAE1, significantly non-inferior to the historical control threshold (14%). MAE included device- or procedure-related death within 30 days post-index procedure, clinically driven target lesion revascularization (cd-TLR) and major index limb amputation up to 12 months post-index procedure.
At 12 months, freedom from cd-TLR was 98.0%, primary patency was 91.2%, survival was 94.8% and freedom from major target limb amputation was 99.5%1. There was clinically relevant improvement in the ankle-brachial index (ABI) 1.
“The BIONETIC-I data confirm that the Dynetic-35 stent is safe and effective in very calcified lesions, which is very important when treating iliac arteries,” said Principal Investigator Prof. Marianne Brodmann, Head of the Clinical Division of Angiology, Department of Internal Medicine at Medical University Graz in Graz, Austria. “Data on performance of cobalt chromium stents in iliac lesions has been scarce, so this is essential to add to the body of evidence.”
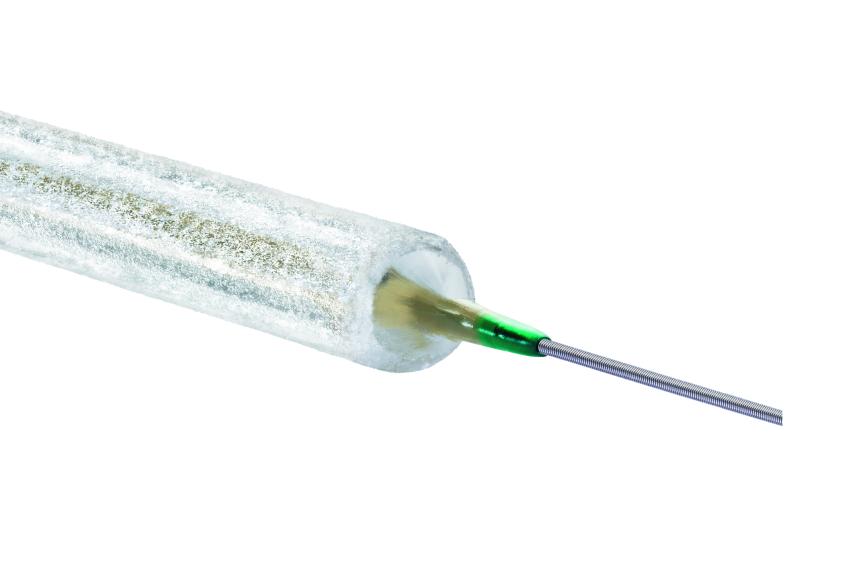
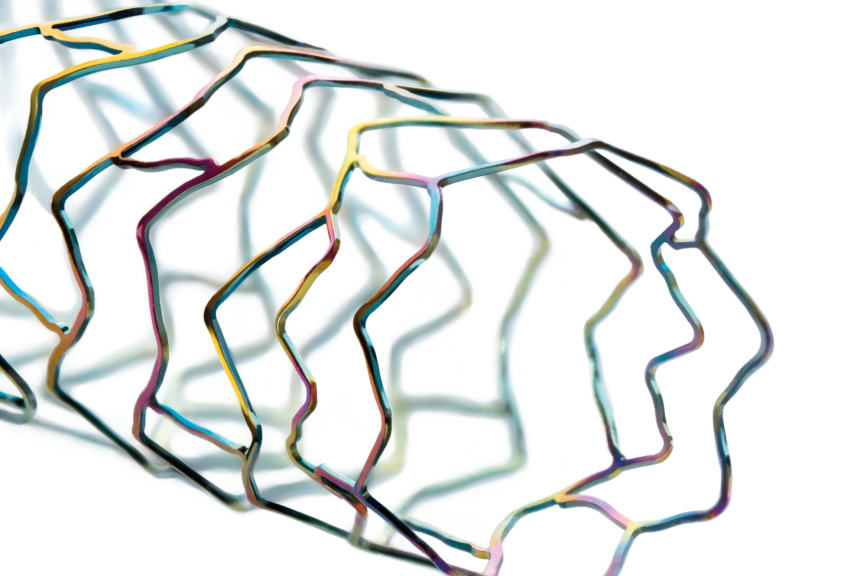
This next-generation Dynetic-35 iliac stent system, which is available in the European Union and other CE mark-accepting countries, is 6F compatible across the entire size matrix, and is indicated for the treatment of de novo or restenotic lesions in the iliac arteries. Furthermore, Dynetic-35 is available with longer catheter lengths designed to allow a transradial approach.
"We are proud to demonstrate the clinical success of our Dynetic-35 iliac stent in an international, multi-center setting," said Dr. Jörg Pochert, President Vascular Intervention at BIOTRONIK. “The features of this device illustrate our commitment to innovation, and now we have evidence to support the utility of this design.”
The BIONETIC-I study will continue collecting follow-up data at 24 and 60 months, which will be shared upon completion of each follow-up period.
-END-
References:
1. Brodmann M. BIOTRONIK Dynetic-35 cobalt chromium balloon-expandable stent for peripheral iliac lesions: 12-month results of the BIONETIC-I Multi-Center study. Presented at: LINC 2024; May 30, 2024; Leipzig, Germany.
Disclaimer:
Dynetic is a trademark or registered trademark of the BIOTRONIK Group of Companies.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.