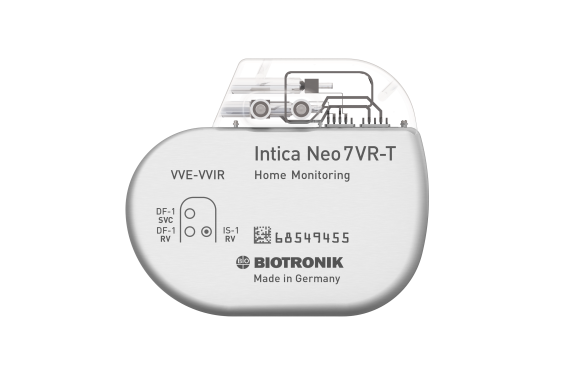
Rivacor 5 HF-T QP/HF-T
Improving treatment and reducing risk for up to 15 years9
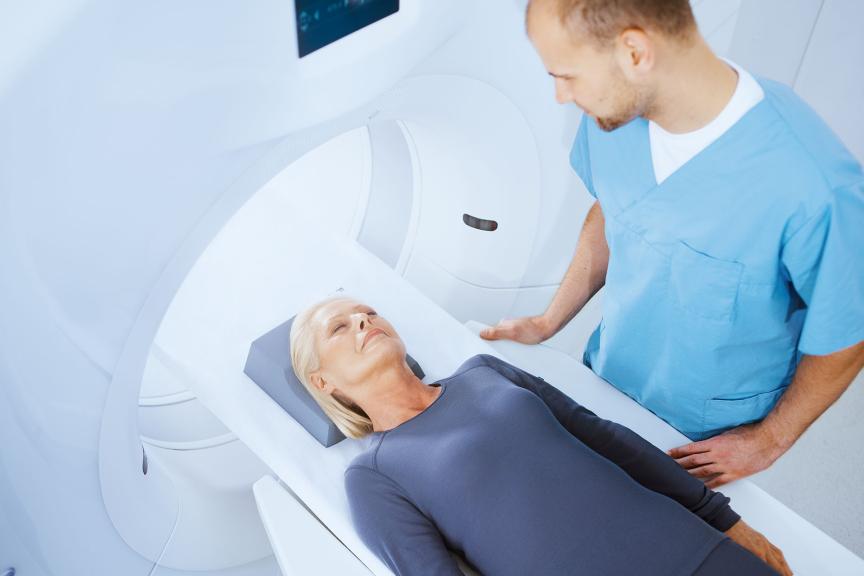
For patients with arrhythmias, ICD therapy can be an essential safety net, where it’s vital to minimize risk and improve quality of life in the long term. This is what the all-new, smaller and simpler Rivacor 5 ICD systems are engineered to do for up to 15 years9 — optimizing therapy when and where it matters. When used with Home Monitoring® technology, AF can be detected earlier14 and both inappropriate shocks and hospitalization rates can be reduced19. And this has been clinically proven.
Product Highlights
BIOshape. Ultraslim 10 mm ICD.
Rivacor ICDs are small and slim – only 10 mm from front to back – with a smooth, elliptical, and body-friendly BIOshape1
15 Years Longevity. 10 Years Warranty.
Rivacor ICDs have an extended battery life of up to 15 years9, supported by a full 10-year warranty
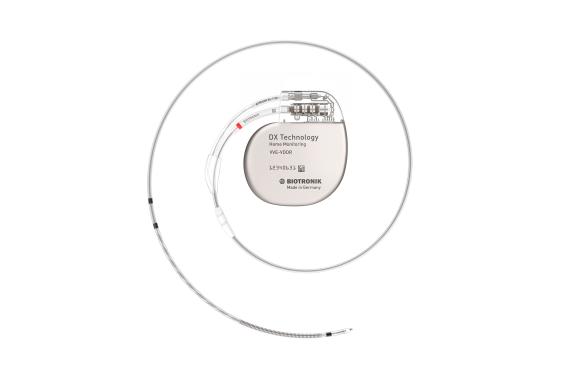
DX Technology
All these benefits are also available with unique DX Technology that provides complete atrial diagnostics with a single lead
Downloads and Related Links
Contact Us
References
1 Contoured housing; Acticor/Rivacor VR: 60 x 61.5 x 10 mm; 30 ccm
2 as part of an MR Conditional system
3 Post-Market observation; interim analysis, December 21, 2018. Data on file
4 Device shape analysis, February 2019. Data on file.
5 Post-market observation; interim analysis, December 21, 2018. Data on file.
6 Post-market observation; interim analysis, December 21, 2018. Data on file.
7 Device shape analysis, February 2019. Data on file.
8 Post-market observation; interim analysis, December 21, 2018. Data on file.
9 Single-Chamber ICD standard conditions. Data on file (service time calculation)
10 Acticor/Rivacor Single-Chamber ICD @ 60 ppm, 15% pacing, 2.5V, 500 Ohms: Medtronic 3T FBS; VISIA AF (EVERA; MIRRO) MRI S VR SureScan:
10.7 years (MDT IFU) vs Acticor 7 VR-T ProMRI: 14.9 years. Competitor device manuals as of Nov. 2018
11 Polyzos KA, Konstantelias AA, Falagas ME, Risk factors for cardiac implantable electronic device infection: A systematic review and meta-analysis,
Europace (2015) 17, 767-777.
12 Fact file: Cardiac Imaging with MRI, CT and Nuclear Techniques British Heart Foundation. January 2010.
13 When patients are monitored by BIOTRONIK Home Monitoring. See ProMRI(R) manual for all details.
14 Varma N et al. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up:
The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) Trial. Circulation, 2010; 122: 325–332.
15 Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial.
J Cardiovasc Electrophysiol, 25 (2014), 763-770.
16 Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial.
J Cardiovasc Electrophysiol, 25 (2014), 763-770.
17 Varma N et al. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up:
The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) Trial. Circulation, 2010; 122: 325–332.
18 Schwab JO et al. Clinical Course of Dual-Chamber Implantable Cardioverter-Defibrillator Recipients followed by Cardiac Remote Monitoring:
Insights from the LION Registry. BioMed Research International, 2018, https://doi.org/10.1155/2018/3120480.
19 Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial.
J Cardiovasc Electrophysiol, 25 (2014), 763-770.
20 Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial.
J Cardiovasc Electrophysiol, 25 (2014), 763-770.
21 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
22 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
23 Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomized controlled trial.
The Lancet. 2014; 384 (9943): 583-590
24 vs. 55% - Crossley G H et al. The CONNEXT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial.
JACC. 2011; 57(10):1181-1189 [for bar chart comparison only]
25 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
26 Performance analysis. Data on file, 2018
27 Performance analysis. Data on file, 2018
28 Kurt M et al. Avoiding inappropriate therapy of single-lead implantable cardioverter defibrillator by atrial-sensing electrodes. Journal of Cardiovasc.
Electrophysiol. 2018; 29(12): 1682-1689