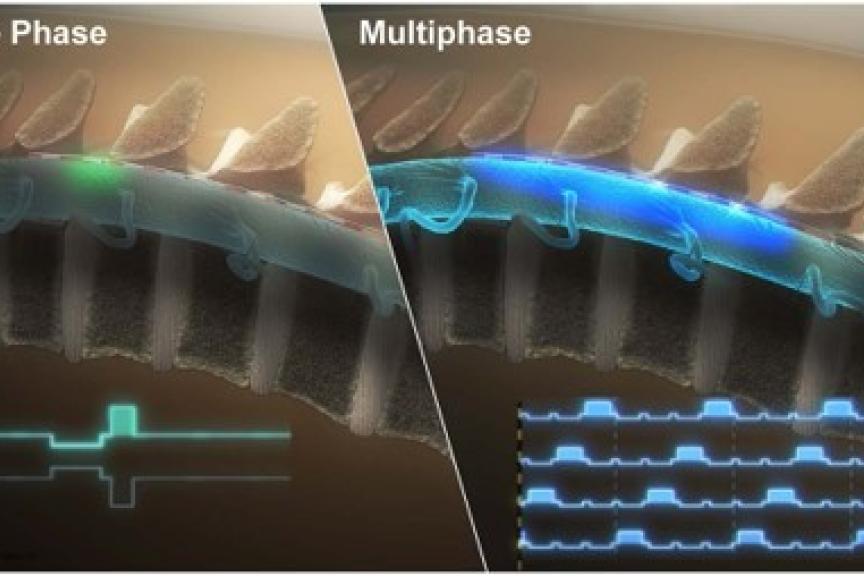
Newly Published Data Supports Effectiveness of BIOTRONIK Neuro’s RESONANCE™ Stimulation BENEFIT-02 Trial Shows that Prospera’s Multiphase Stimulation Paradigm Offers Effective Pain Relief with Lower Power Requirements
Results from the BENEFIT-02 trial – the first of its kind to clinically evaluate a multiphase stimulation paradigm – support the effectiveness of RESONANCE multiphase stimulation used in the BIOTRONIK Neuro Prospera™ Spinal Cord Stimulation (SCS) System in the treatment of patients with chronic pain. In contrast to other currently available SCS therapies, RESONANCE requires less power and uses a proprietary integrated circuit design to deliver a continuous, spatially and temporally distributed therapeutic pulse pattern across the spinal cord. Results of the study were recently published in Neuromodulation.1
The prospective, multicenter, randomized, single-blind, feasibility study assessed the safety and effectiveness of BIOTRONIK’s new charge-distributed multiphase stimulation paradigm during an extended SCS trial. It included participants with chronic low back and/or leg pain and baseline numerical rating scale (NRS) for overall pain intensity ≥6. After a successful commercial SCS trial, participants were randomized to multiphase SCS therapy (A: approx. 600–1500 Hz or B: approx. 300–600 Hz). Following washout, therapy was delivered via an investigational external pulse generator and existing leads during an 11- to 12–day testing period.
Among the 65 patients completing the study, there was no statistically significant difference in mean NRS reduction or percent pain relief between multiphase therapies A and B. In the at-home setting, 63.9% of participants reported greater pain relief with multiphase than with commercial SCS therapy, along with an increase in average sleep quality and physical activity. Additionally, adverse events were rare, and none were classified by investigators as serious. On average, multiphase required less power than did commercial therapies.1
BENEFIT-02 is part of a comprehensive research program that supports BIOTRONIK’s Prospera SCS System, which received FDA approval in March 2023. Prospera not only features the first and only RESONANCE multiphase stimulation paradigm, it also includes Embrace One™ – a patient-centric care model that enables proactive care* through automatic, objective, daily remote monitoring.
“Former studies have shown good results with previously commercialized SCS technology, yet in practice we are still looking for ways to reduce patient burden and improve outcomes,” said Dr. Leonardo Kapural, pain physician at the Carolinas Pain Institute and Center for Clinical Research in Winston-Salem, North Carolina, USA and Principal Investigator of the BENEFIT-02 study. “This study is particularly exciting because it’s the first in a series to explore how to do that. The short-term multiphase stimulation results here – combined with early findings from the long-term BENEFIT-03 study – show great promise for the future of SCS.”
BENEFIT-03 is the first long-term evaluation of the Prospera SCS system with RESONANCE multiphase stimulation; automatic, daily, objective, device monitoring; and remote programming. Interim results from the ongoing study in Australia support BIOTRONIK Neuro’s proactive care approach to SCS therapy. Interim six-month results recently presented at the ASPN Annual Conference demonstrated significant pain reduction in both in-clinic and at-home settings with less severe disability.2
“As we await additional data from our BENEFIT-03 study, we’re excited to see these initial data on the long-term effects of our SCS system,” said BIOTRONIK Neuro President Todd Langevin. “We developed Prospera to offer patients sustainable pain relief – and we believe its proactive care model will have a clinically meaningful impact on lowering long-term failure rates and reducing the service burden of SCS.”
-END-
References:
1 Kapural L, Patterson DG, Li S, Hatheway J, Hunter C, Rosen S, Fishman M, Gupta M, Sayed D, Christopher A, Burgher A, McJunkin T, Ross EL, Provenzano D, Amirdelfan K. Multiphase Spinal Cord Stimulation in Participants With Chronic Back or Leg Pain: Results of the BENEFIT-02 Randomized Clinical Trial, Neuromodulation: Technology at the Neural Interface, 2023, https://doi.org/10.1016/j.neurom.2023.05.006.
2 Russo M, Yu J, Mohabbati V, Amirdelfan K, Kapural L, Verrills P. An implantable SCS system with multiphase stimulation and remote device management: Interim 6-month study results. Poster presented at: The American Society of Pain & Neuroscience July 13-16, 2023; Miami Beach, FL.
Prospera SCS system is not CE marked and not available for sale in CE accepting markets or other geographies outside of the United States.
Embrace One is a support platform intended to help manage a patient’s experience with spinal cord stimulation. It is not intended to be used for medical diagnosis or medical treatment.
*BIOTRONIK Neuro’s remote support team may reach out to patients to ensure proper usage of the spinal cord stimulator based on remotely monitored data. BIOTRONIK Neuro does not provide health advice or clinical actions outside the scope of spinal cord stimulator proper usage. This product support is not a replacement for the patient’s responsibility to communicate any medical questions or concerns with the physician’s office.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for more than 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.