BENEFIT-01 Study Now Published in Neuromodulation: A Glimpse into the Future of Spinal Cord Stimulation Programming BIOTRONIK Neuro’s Pioneering Study Unveils Crucial Insights into Sub-perception SCS Therapy, Shaping Future Innovations in Chronic Pain Management
BIOTRONIK is pleased to announce the release of the results of the BENEFIT-01 study published in Neuromodulation.1 The prospective, multi-center study was designed to investigate the influence of clinically relevant Spinal Cord Stimulation (SCS) parameters on patient perception. BENEFIT-01 marked the beginning of a series of SCS clinical studies with the aim of developing an SCS technology that provides sustained pain relief with few side effects.
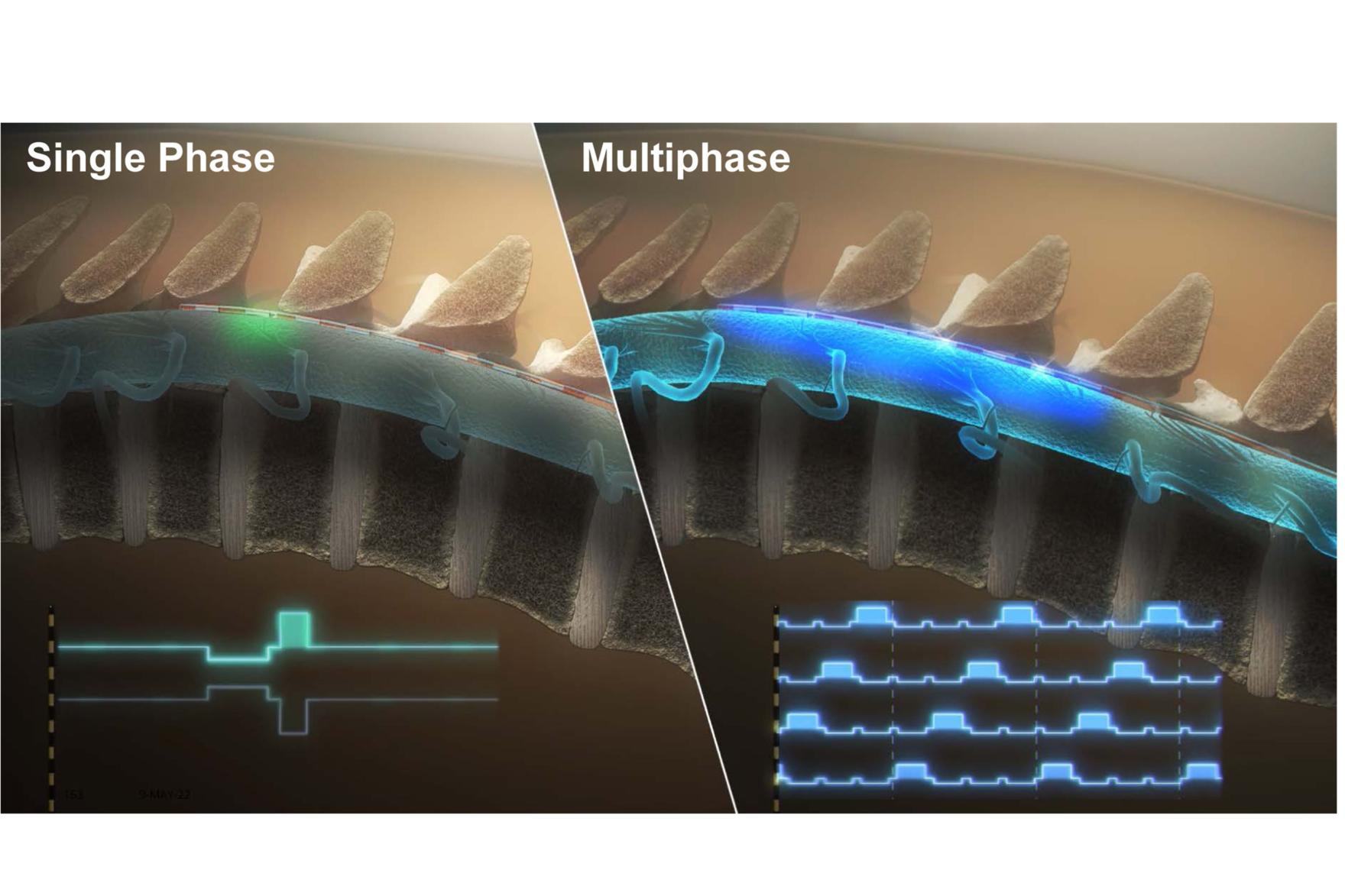
From July to November 2017, 40 patients with low back and/or leg pain who had completed a commercial SCS trial were enrolled at seven clinical sites in the US and underwent approximately 90 minutes of test stimulation prior to trial lead removal. The analysis of this BENEFIT-01 data led to the creation of a map defining the boundary of patient perception of stimulation and revealed that SCS sensations are mainly influenced by pulse width, not frequency. The study also explored alternative electrode configurations, offering valuable insights for improving SCS outcomes.
“This was an incredible opportunity to explore some of the challenges facing conventional SCS therapies,” noted Dr. John Hatheway, the lead author of BENEFIT-01. “These findings advance our understanding of the therapy and have helped pave the way for proprietary stimulation paradigms like RESONANCE™.”
This work represents one aspect of a comprehensive research program supporting the Prospera™ SCS system and BIOTRONIK Neuro’s proprietary multiphase stimulation paradigm, RESONANCE. BIOTRONIK Neuro developed this technology by integrating the results from BENEFIT-01 with SCS modelling, pre-clinical animal studies, and results from BENEFIT-02, a subsequent clinical study that evaluated the short-term safety and effectiveness of multiphase stimulation.
“BENEFIT-01 was the first in a series of studies that speaks to our commitment to advancing and sharing the science behind SCS,” said Todd Langevin, BIOTRONIK Neuro President. “It’s exciting to see these learnings realized as patients experience the positive impact of RESONANCE™ stimulation with our Prospera™ SCS system.”
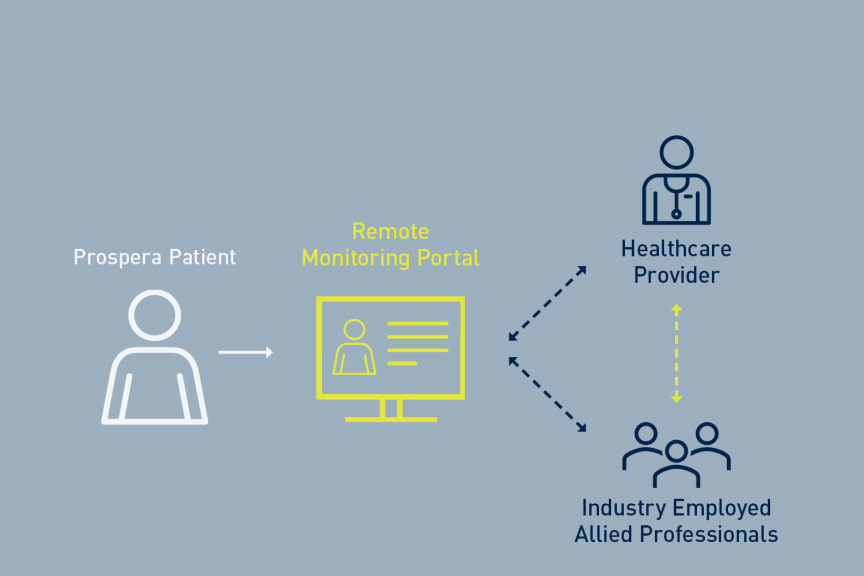
Results from BENEFIT-02, published in Neuromodulation in October 2023, highlighted the effectiveness of Prospera's Multiphase Stimulation Paradigm, demonstrating its efficacy in pain relief with lower power requirements.2 Building on these early clinical and pre-clinical results, the BENEFIT-03 study, ongoing in Australia, is evaluating the long-term safety and effectiveness of the Prospera SCS System with RESONANCE and introduced automatic daily device monitoring and remote programming. Interim 12-month results from BENEFIT-03 were presented at the 2024 North American Neuromodulation Society conference last week.3
-END-
References:
1. Hatheway J, Yang M, Fishman M, Verdolin M, McJunkin T, Rosen S, Slee, S, Kibler A, Amirdelfan K. Defining the Boundaries of Patient Perception in Spinal Cord Stimulation Programming. Neuromodulation 2024: 1-10.
2. Kapural L, Patterson DG, Li S, Hatheway J, Hunter C, Rosen S, Fishman M, Gupta M, Sayed D, Christopher A, Burgher A, McJunkin T, Ross EL, Provenzano D, Amirdelfan K. Multiphase Spinal Cord Stimulation in Participants With Chronic Back or Leg Pain: Results of the BENEFIT-02 Randomized Clinical Trial. Neuromodulation 2023: 1-12.
3. Russo M, Yu J, Mohabbati V, Amirdelfan K, Kapural L, Verrills P. Long-Term Study of SCS System with Multiphase Stimulation and Remote Device Management: Interim 12-Month Results. Poster presented at: North American Neuromodulation Society Annual Conference; January 18, 2024; Las Vegas, NV.
Disclaimer:
Prospera SCS system is not CE marked and not available for sale in CE accepting markets or other geographies outside of the United States.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.