First Patient Enrolled in Leave Nothing Behind-Trial Comparing Drug-Coated Balloon-Based Strategy to Drug-Eluting Stents in Chronic Total Occlusion Patients The Randomized Clinical Trial Aims to Investigate and Improve the Treatment of Chronic Total Occlusions
Today BIOTRONIK announces the enrollment of the first patient in the Leave Nothing Behind-Trial. The first implantation was performed by Dr. Mohamed Ayoub at the Heart and Diabetes Center North Rhine-Westphalia, Germany.
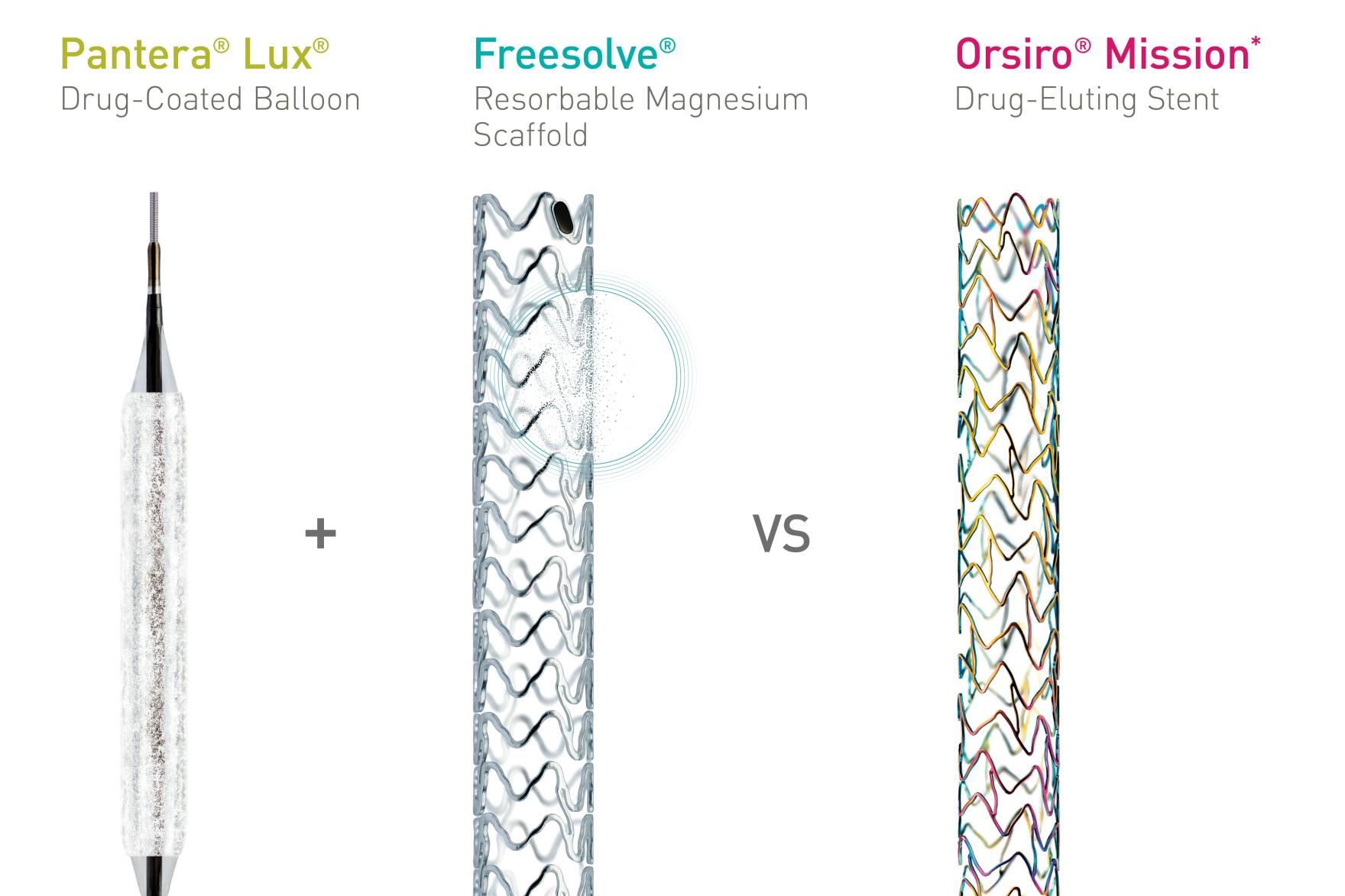
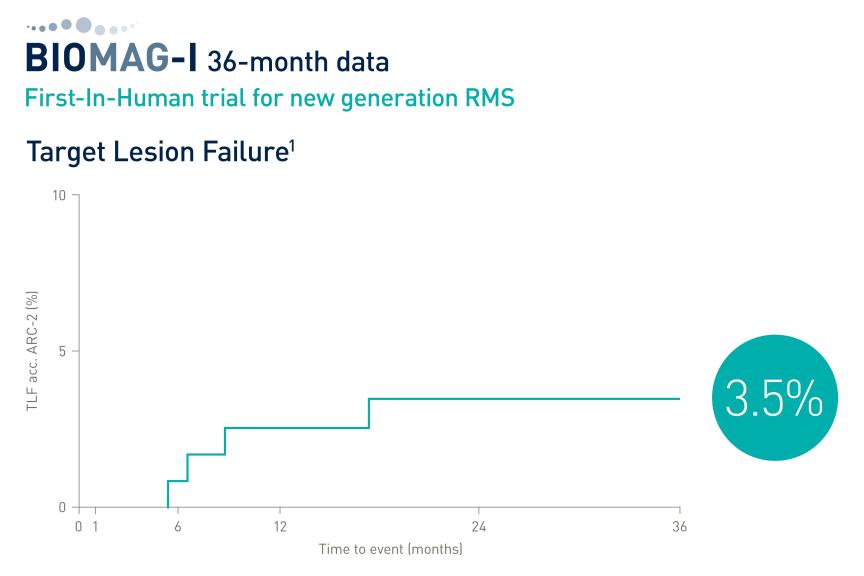
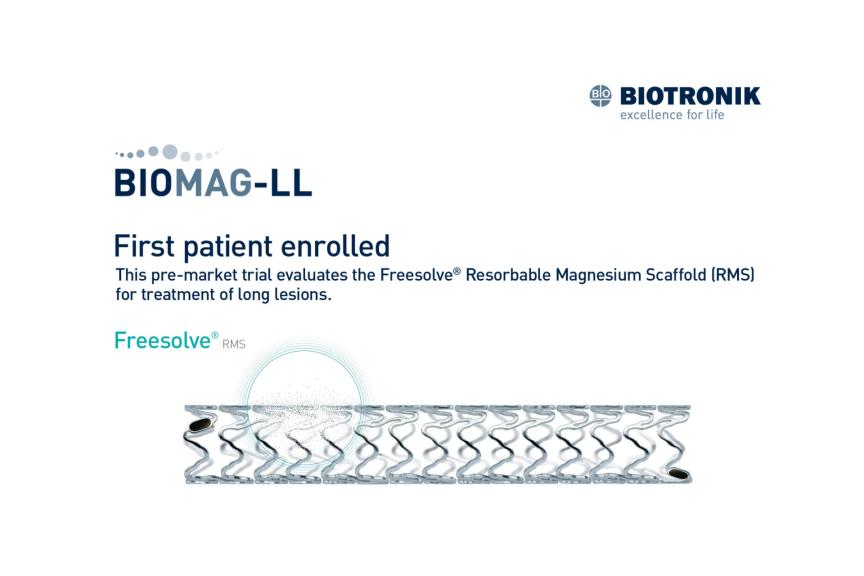
The trial aims to demonstrate the non-inferiority of drug-coated balloons (DCB) or DCBs in combination with Resorbable Magnesium Scaffolds (RMS) compared to drug-eluting stents (DES) in chronic total occlusions (CTO) in percutaneous coronary interventions (PCI). The prospective, single-center, single-blind, randomized trial evaluates the efficacy of the Pantera® Lux® DCB and Freesolve® RMS compared to Orsiro® Mission* DES in 166 CTO patients.
“We want to investigate whether treatment with DCBs ─ used alone or with Resorbable Magnesium Scaffolds as a bailout option ─ is non-inferior to drug-eluting stents in patients with chronic total occlusions,” summarized Principal Investigator Dr. Mohamed Ayoub, Heart and Diabetes Center North Rhine-Westphalia, Bad Oeynhausen, Germany. “If the study provides evidence in favor of this approach, patients with CTOs or complex lesions could be treated with DCBs or Freesolve RMS on a routine base with excellent results.”
The Leave Nothing Behind-Trial targets an angiographically well-defined group of patients with CTO and J-CTO scores ranging from 0 to 3. Patients will be randomized in a 1:1 treatment allocation between DCB/DCB+RMS and DES. Primary endpoint is the average efficacy of the treatment approach at nine months post-implantation. Clinical endpoints, including major adverse cardiac events, at nine-month and three-year follow-ups constitute the secondary endpoint.
“We are excited to embark on this trial that represents a significant step forward in the quest to improve patient outcomes and quality of life”, said Prof. Dr. Georg Nollert, Vice President Medical Affairs, Vascular Intervention at BIOTRONIK. “While Chronic Total Occlusions are constantly increasing, the use of advanced technologies like the Pantera Lux DCB and Freesolve RMS could pave the way for new standards in CTO treatment, by leaving nothing behind for future interventions."
-END-
References:
* Clinical data collected with Synsiro® Pro Drug-Eluting Stent. Synsiro® Pro Drug-Eluting Stent is part of the Orsiro® Family of DES and can be used to illustrate Orsiro® Mission Drug-Eluting Stent clinical safety and performance.
For more information, please visit: BIOTRONIK What would you choose?
Freesolve® RMS is available in CE-mark accepting countries
Pantera, Lux, Freesolve, Orsiro, Orsiro Mission and Synsiro are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries across the Americas, EMEA (Europe, the Middle East, and Africa), and Asia-Pacific.