New BIOlMASTER.Selectra 3D Study Highlights Exceptional Safety and Performance of BIOTRONIK’s Selectra 3D Guiding Catheter for CSP Selectra 3D Is Specifically Designed to Reliably Support Conduction System Pacing (CSP) Procedures
In a recent prospective, international, multicenter, nonrandomized trial, the BIO|MASTER.Selectra 3D study assessed the performance and safety of BIOTRONIK’s Selectra 3D guiding catheter, revealing compelling insights into its ease of handling and effectiveness. Conducted across 10 centers in Australia, Hong Kong and Europe, the study included 157 patients who underwent a CSP procedure for bradycardia pacing or heart failure indications. The results of this research have been published in the Heart Rhythm O2 journal.1
Key Findings of the BIO|MASTER.Selectra 3D Study:
- High Success Rate in Left Bundle Branch Area Pacing (LBBAP) and Overall for CSP Procedures: The use of Selectra 3D catheters demonstrated an impressive 99% success rate in LBBAP implantations and 94% for CSP in general.
- Positive Physician Feedback: Implanting physicians provided 94% positive feedback for stylet-supported implantations, achieved with the combination of the Selectra 3D catheter and the Solia S lead.*
- Exceptional Handling: Implanters rated the catheter’s handling characteristics for direct LBBAP approach very positively – namely, more than 95%.
- Flexibility to Change Between Different Approaches: Selectra 3D catheters successfully accommodate CSP procedures and offer the flexibility to switch between different approaches and techniques when tackling challenging areas.
Professor Dr. Jan De Pooter, a leading expert in CSP from the University Hospital Ghent, Belgium, and the Principal Investigator of the study, commented on the findings, emphasizing the role of CSP tools in the rapidly evolving LBBAP landscape. He said, “The BIO|MASTER.Selectra 3D study highlights the crucial role of the Selectra 3D guiding catheter in both His-bundle pacing (HBP) and LBBAP implantations. This catheter is associated not only with high implant success, but also with an excellent safety profile, characterized by an outstanding 100 percent absence of adverse events seven days postimplantation. With the clinical relevance of CSP increasing day by day, well-performing tools like Selectra 3D become indispensable for many implanters.”
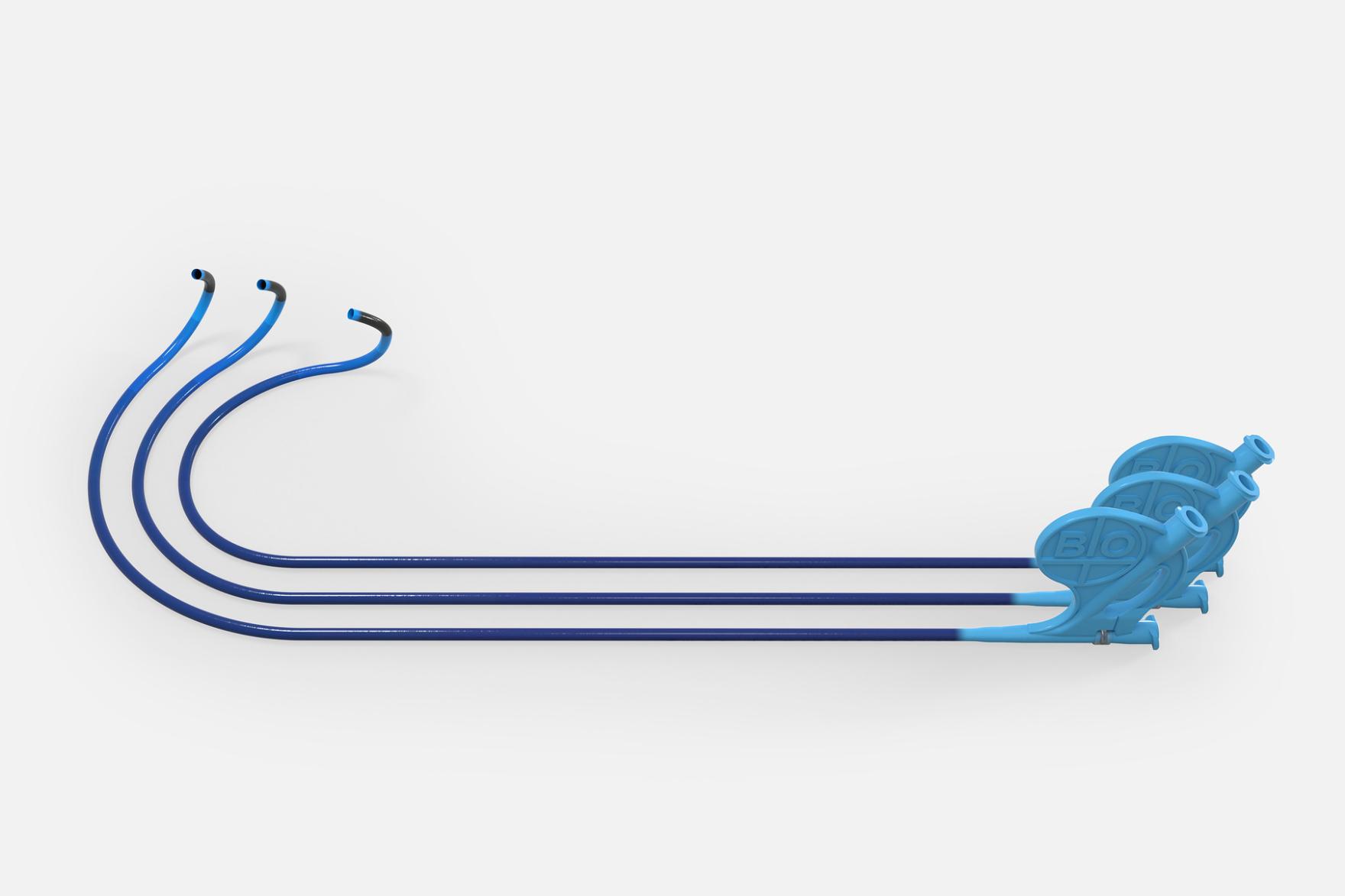
The Selectra 3D guiding catheter is specifically designed to ease the positioning of stylet-driven leads during CSP procedures, which can be challenging. Effectively, this catheter provides control for placement of the pacing lead onto the targeted site and adapts to different patient profiles.
“Selectra 3D stands out as the state-of-the-art guiding catheter for CSP procedures, with excellent handling characteristics,” expressed Dr. Francesco Zanon, coauthor of the BIO|MASTER.Selectra 3D study from the Santa Maria della Misericordia General Hospital, Rovigo, Italy. He further emphasized its versatility, stating: "By offering a wide range of curves and more lengths, it’s a tool that gives cardiologists more choices and flexibility to address various patient anatomies and different procedural requirements during implantations.”
Dr. Andreas Hecker, President CRM/EP at BIOTRONIK, underscored BIOTRONIK’s leadership in the CSP field: “With our unique conduction system pacing tools, excellent technical support, and educational training programs, BIOTRONIK remains at the forefront of advancements in CSP. We take pride in being a trusted partner for the medical community in this emerging field and look forward to continuing our close collaboration with physicians to further advance BIOTRONIK’s CSP solution, intended to facilitate CSP approaches and improve patient care.”
The BIO|MASTER.Selectra 3D study results will be presented during a dedicated CSP symposium at the EHRA Congress, which is taking place from April 7–9, 2024, in Berlin.
-END-
References:
De Pooter, Jan; Bulava, Alan; Gras, Daniel et al. (2023): Utility of a guiding catheter for conduction system pacing: an early multicenter experience, Heart Rhythm O2, 2023, ISSN 2666-5018, https://doi.org/10.1016/j.hroo.2023.11.017
* Solia S lead is not approved for CSP indications.
Disclaimer:
Selectra 3D and Solia S are not currently approved for CSP indications in the United States. Content is not intended for United States healthcare providers.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.