BIOFLOW-II
NCT01356888
Estudo do sistema de stent farmacológico Orsiro
Conclusão
- Falha de lesão alvo (TLF) comparável a Xience Prime *, embora se separando ao longo do tempo em favor de Orsiro em 48 meses
- Ausência de trombose de stent definitiva ou provável também em populações de alto risco, como diabéticos e subgrupo de pequenos vasos até 48 meses
- Os resultados deste estudo prospectivo e randomizado confirmam a segurança e eficácia do Orsiro
Design de estudo
Um estudo prospectivo, multicêntrico, randomizado e controlado comparando o Orsiro DES com o Xience PrimeCoordenando investigadores clínicos: Prof. Stephan Windecker, Bern, Suíça e Dr. Thierry Lefèvre, Massy, França. Endpoint primário: Perda tardia de lúmen no Stent (LLL) em 9 meses.
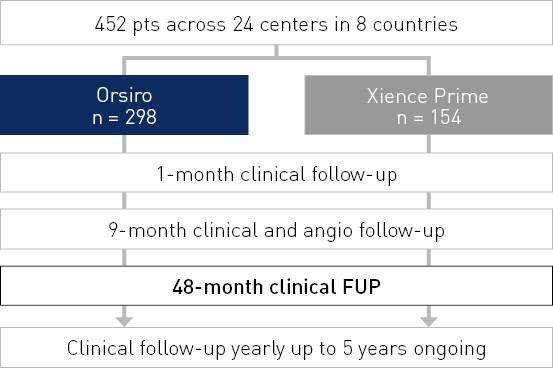
Resultados em 48 meses
Taxa TLF - todas as disciplinas
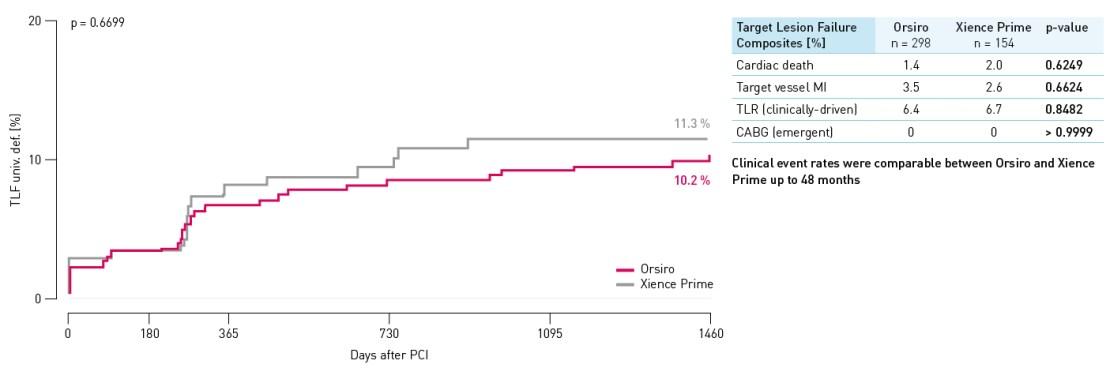
Taxa TLF - população de pequenos navios
TLF definido como composto de morte cardíaca, onda Q do vaso alvo ou infarto do miocárdio (MI) não onda Q, enxerto de bypass da artéria coronária (CABG) e TLR clinicamente conduzido.
Todos os resultados de trombose de stent do sujeito em 48 meses. Nenhuma trombose de stent definida e provável ocorreu em nenhum dos braços por 48 meses


Isenção de responsabilidade
© BIOTRONIK AG - Todos os direitos reservados.
As especificações estão sujeitas a modificações, revisões e melhorias.
* Xience e Xience Prime são marcas registradas da Abbott Cardiovascular Systems.
Referência: Ton Slagboom em nome dos Investigadores BIOFLOW-II, pôster, euroPCR 2017
