Vascular Intervention // Peripheral
Balloon-Expandable Cobalt Chromium
Stent System / 0.035" / OTW
The next generation iliac stent with excellent
radial strength and superb flexibility.











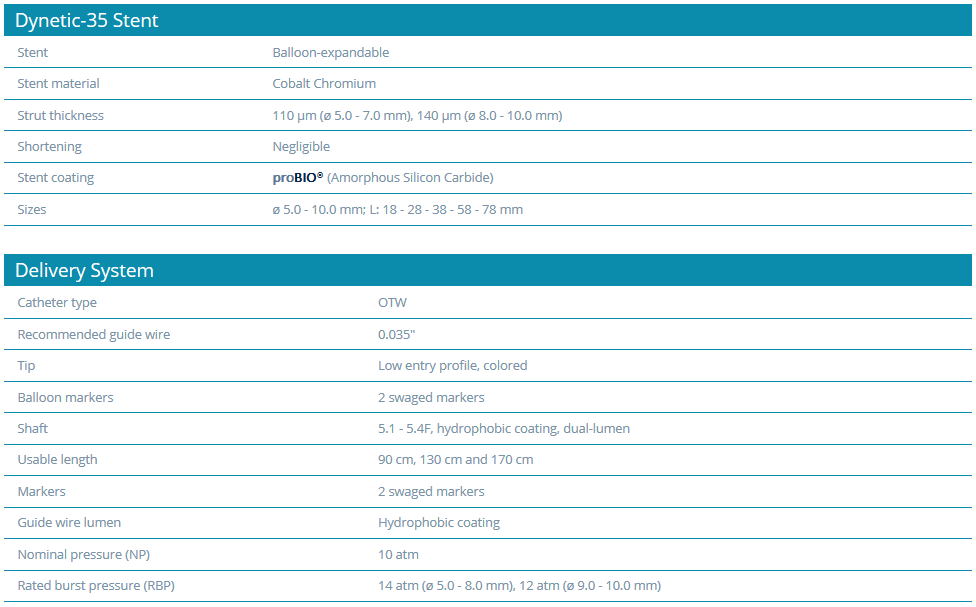
Technical Data
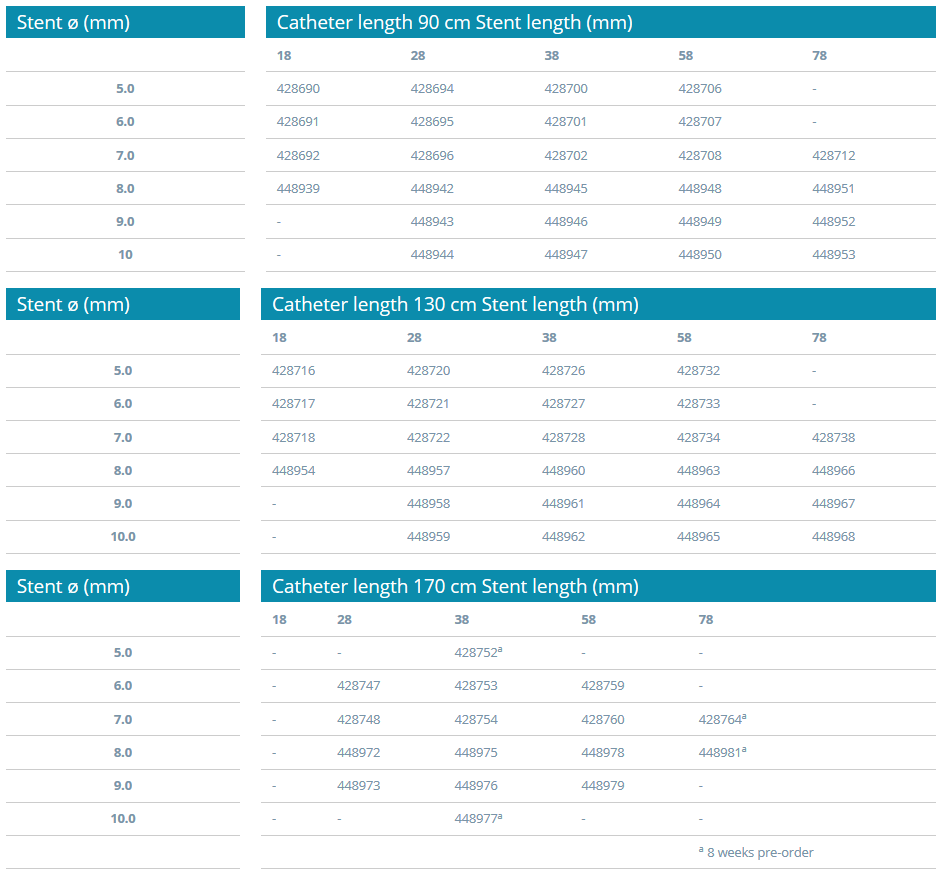
Ordering Information
Contact
1. BIOTRONIK data on file. 8.0 mm stent diameter; 2. BIOTRONIK data on file; 3. Endovascular Today – Europe Buyer´s Guide 2019, Balloon-Expandable Stents. http://evtoday.com/device-guide/european/152#; 4. BIOTRONIK data on file. IIB(P) 13-2019. 10.0 mm stent diameter; 5. BIOTRONIK data on file. IIB(P)13-2019. 8.0 mm stent diameter; 6. BIOTRONIK data on file. IIB(R) 20-2005. 8.0 mm stent diameter.
**Indication as per IFU.
††Note: The Dynetic-35 does not have TGA (Therapeutic Goods Administration – Australia) approval for use within the common iliac artery.
Dynetic and proBIO are trademarks or registered trademarks of the BIOTRONIK Group of Companies. Visi-Pro is a trademark or registered trademark of the Medtronic Group of companies. Flexive and Express are trademarks or registered trademarks of the Boston Scientific Group of companies. OmniLink and OmniLink Elite are trademarks or registered trademarks of the Abbott Group of companies. Isthmus Logic is a trademark of the Alvimedica Group of companies.




