Vascular Intervention // Peripheral
PTA Balloon Catheter/0.035"/OTW
Low profile PTA balloon with excellent deliverability
in a wide range of sizes.









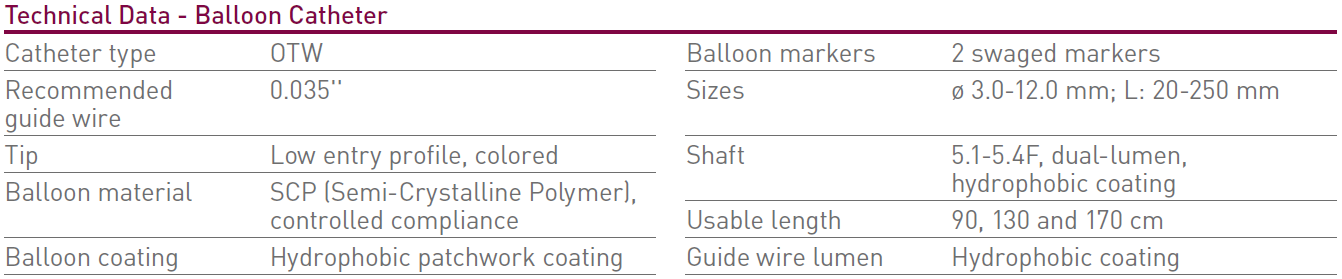
Technical Data
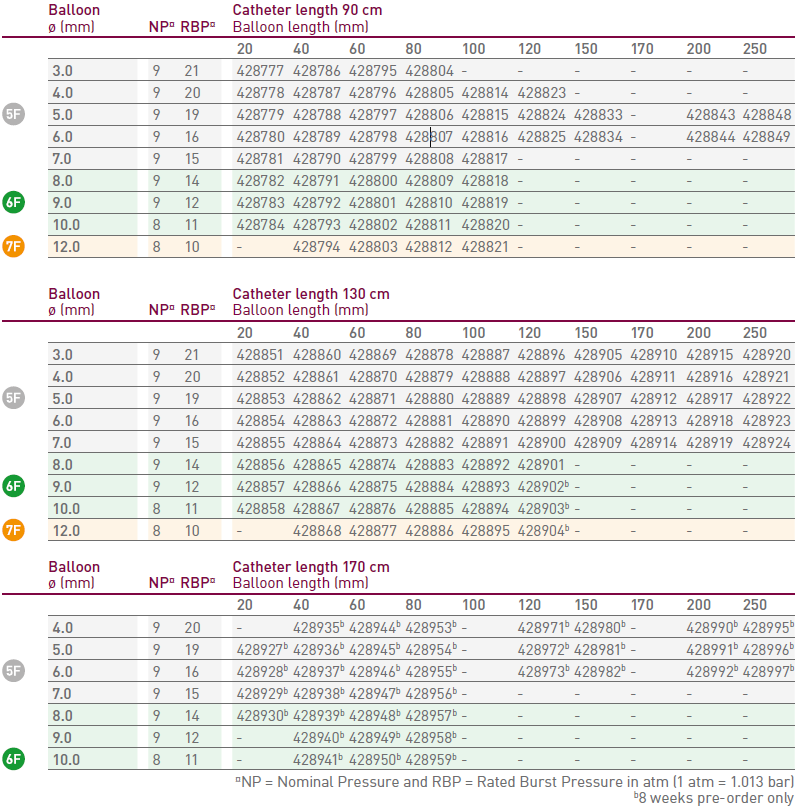
Compliance and Ordering Information
1. BIOTRONIK data on file. Compared to leading competitors 6.0 mm diameter balloon size. IIB(P) 39/2017; 2. BIOTRONIK data on file. Compared to leading competitors 6.0x200 mm balloon size. *Note for Australia: Passeo-35 Xeo not approved by Therapeutic Goods Administration for use in the common iliac arteries.**Indication as per IFU. Passeo and Xeo are trademarks or registered trademarks of the BIOTRONIK Group of Companies. EverCross is a trademark or registered trademark of the Medtronic Group of companies. Mustang is a trademark or registered trademark of the Boston Scientific Group of companies. Ultraverse is a trademark or registered trademark of Becton, Dickinson and Company or its affiliates. Armada is a trademark or registered trademark of the Abbott Group of companies.




